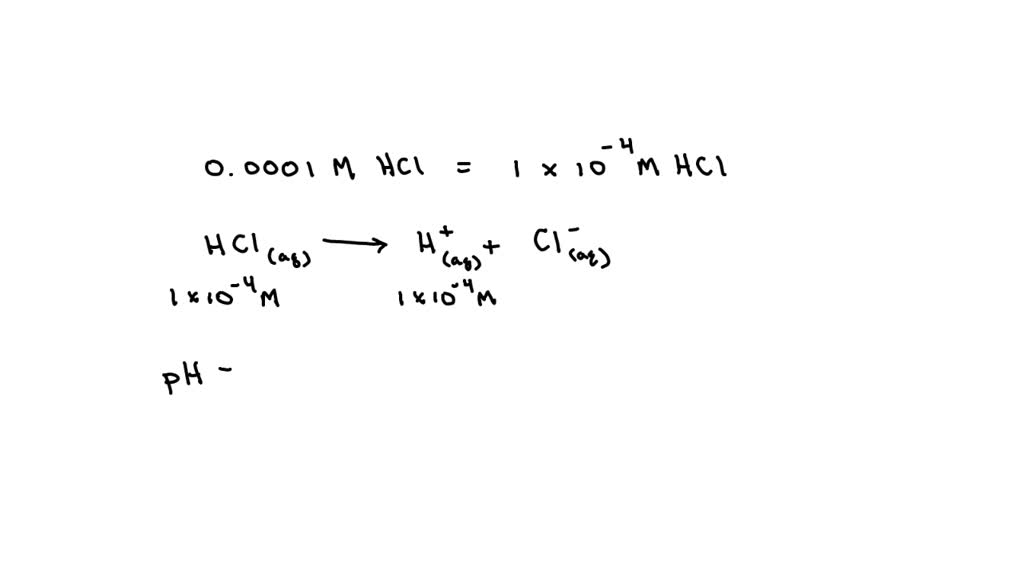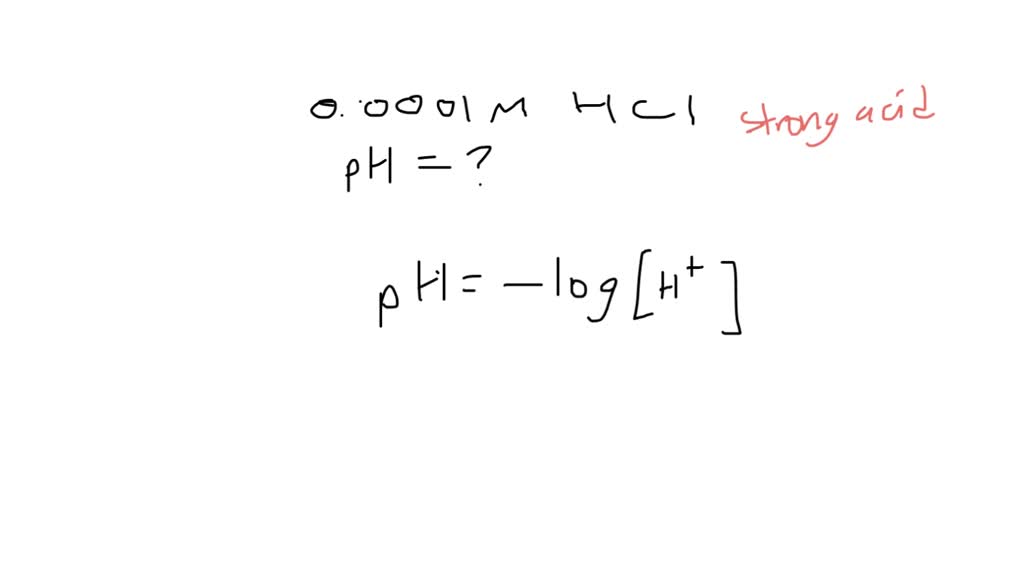What Is The Ph Of A 0 0001 M Hcl Solution
What Is The Ph Of A 0 0001 M Hcl Solution - The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? What is the ph of a 0.0001m hcl solution? The ph of 0.0001 n solution of koh will be: What is the ph of a soution containing.0001 m hcl? (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of 0.0001m hcl solution? Our expert help has broken down your. How ph value is increased when hcl acid solution is diluted. A lab assistant prepared a solution by adding a.
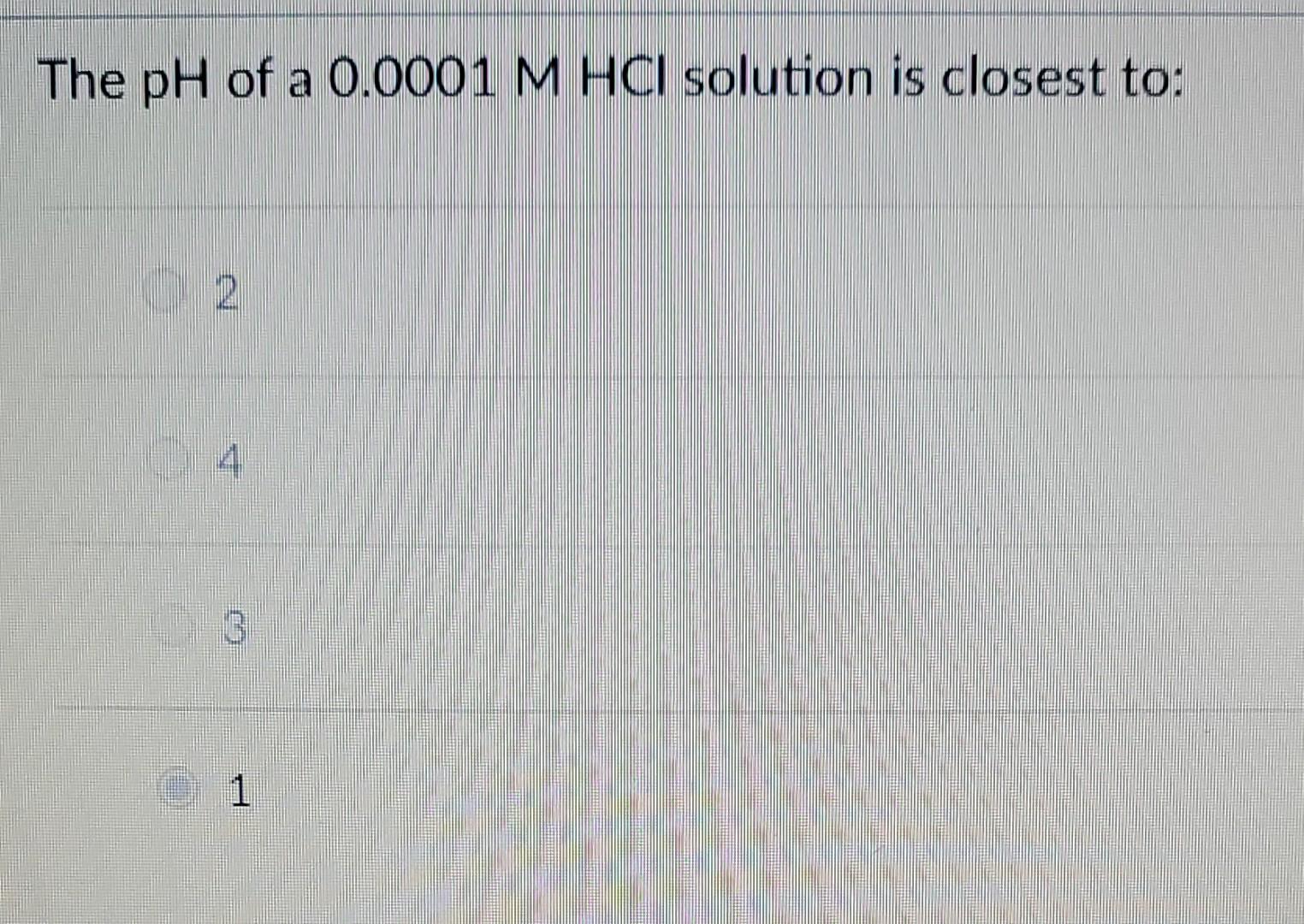
The ph of 0.0001 n solution of koh will be: Our expert help has broken down your. A lab assistant prepared a solution by adding a. What is the ph of a soution containing.0001 m hcl? The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of a 0.0001m hcl solution? (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of 0.0001m hcl solution? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. How ph value is increased when hcl acid solution is diluted.
(remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? How ph value is increased when hcl acid solution is diluted. Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. What is the ph of 0.0001m hcl solution? What is the ph of a 0.0001m hcl solution? A lab assistant prepared a solution by adding a. The ph of 0.0001 n solution of koh will be: Our expert help has broken down your. Aqueous solution of hcl has the ph = 4.
Solved What is the pH of a solution that is 0.0001 M HCl?
What is the ph of 0.0001m hcl solution? The ph of 0.0001 n solution of koh will be: Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. Aqueous solution of hcl has the ph = 4. What is the ph of a soution containing.0001 m hcl?
pH of 1M HCl i
Our expert help has broken down your. Aqueous solution of hcl has the ph = 4. What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? What is the ph of a soution containing.0001 m hcl? The ph of a solution is determined by taking the negative log of.
Find the pH of a 0.025 HCl (Hydrochloric acid) Solution YouTube
The ph of 0.0001 n solution of koh will be: Aqueous solution of hcl has the ph = 4. What is the ph of a 0.0001m hcl solution? The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it.
The pH value of 10 M solution of HCl is
Aqueous solution of hcl has the ph = 4. How ph value is increased when hcl acid solution is diluted. The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. What is the ph of a 0.0001m hcl solution? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that.
1 M TrisHCl (pH 7.4), (ML01774) Welgene
(remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of a soution containing.0001 m hcl? The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions. How ph value is increased when hcl acid solution is diluted. What is the ph.
Solved Help Please! 1)Explain The Reasons For The Differe...
What is the ph of 0.0001m hcl solution? What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? Aqueous solution of hcl has the ph = 4. (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! Ph = 4.0 hydrochloric acid,.
SOLVED Calculate the pOH of a 0.0001 M HCl solution (Express your
Our expert help has broken down your. What is the ph of a soution containing.0001 m hcl? What is the ph of 0.0001m hcl solution? How ph value is increased when hcl acid solution is diluted. The ph of a solution is determined by taking the negative log of the concentration of hydrogen ions.
what is the pH of 0.0001 M HCl solution
What is the ph of 0.0001m hcl solution? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. A lab assistant prepared a solution by adding a. The ph of 0.0001 n solution of koh will be: How ph value is increased when hcl acid solution is diluted.
SOLVED Calculate the pH of a 0.0001 M HCl solution (Express your
What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. Aqueous solution of hcl has the ph = 4. (remember hcl is a strong acid and therefore dissociates.
The Ph Of A Solution Is Determined By Taking The Negative Log Of The Concentration Of Hydrogen Ions.
What is the ph of a 0.0001m hcl solution? The ph of 0.0001 n solution of koh will be: (remember hcl is a strong acid and therefore dissociates completely in water) your solution’s ready to go! What is the ph of 0.0001m hcl solution?
Our Expert Help Has Broken Down Your.
Aqueous solution of hcl has the ph = 4. What is the ph of a soution containing.0001 m hcl? Ph = 4.0 hydrochloric acid, hcl, is a strong acid, which means that it dissociates completely in aqueous solution to produce. What is the ph of the resulting solution when equal volumes of 0.1 m naoh and 0.01 m hcl are mixed?
A Lab Assistant Prepared A Solution By Adding A.
How ph value is increased when hcl acid solution is diluted.