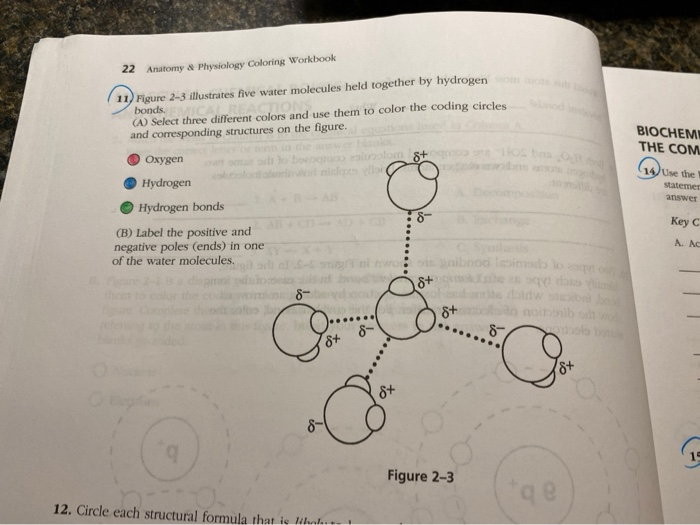
What Does The Dotted Line Between The Water Molecules Represent
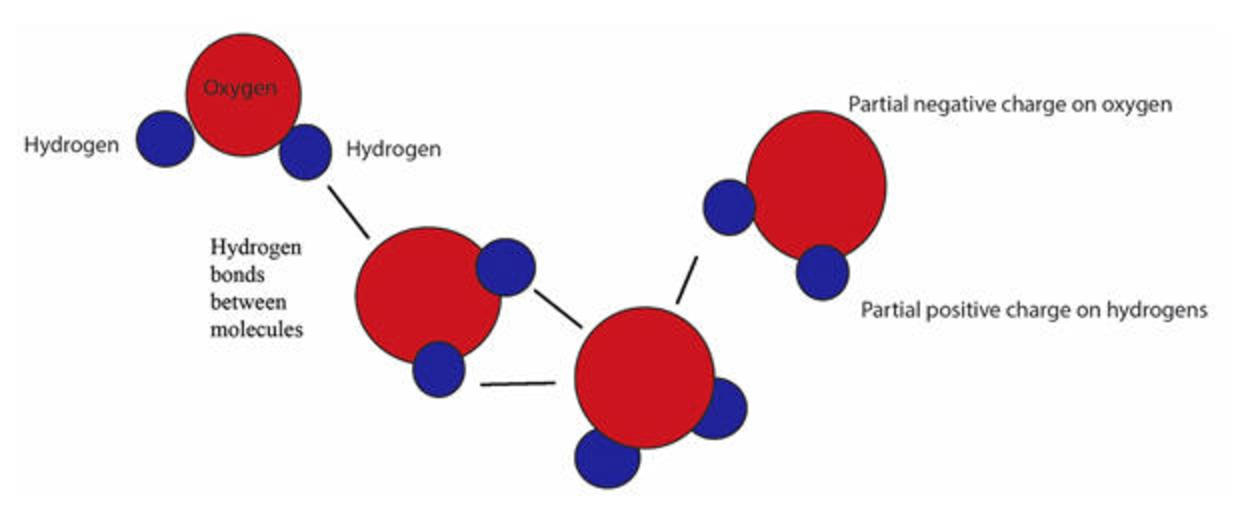
What Does The Dotted Line Between The Water Molecules Represent - The force of attraction, shown here as a dotted line, is called a hydrogen bond. Hydrogen bonds shown as the dotted lines between water molecules. Each water molecule is hydrogen bonded to four. In the case of water, hydrogen bonds form between. What does the dotted line between the water molecules represent? The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. A polar bond a covalent bond a. This type of bond is a special. The diagram shows two water molecules. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond.
Each water molecule is hydrogen bonded to four. In the case of water, hydrogen bonds form between. The diagram shows two water molecules. Hydrogen bonds shown as the dotted lines between water molecules. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. The force of attraction, shown here as a dotted line, is called a hydrogen bond. This type of bond is a special. A polar bond a covalent bond a. What does the dotted line between the water molecules represent? The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen.
This type of bond is a special. What does the dotted line between the water molecules represent? The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. In the case of water, hydrogen bonds form between. The diagram shows two water molecules. The force of attraction, shown here as a dotted line, is called a hydrogen bond. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. Each water molecule is hydrogen bonded to four. A polar bond a covalent bond a. Hydrogen bonds shown as the dotted lines between water molecules.
Task Draw water molecules interacting with each possible p.pdf
This type of bond is a special. The diagram shows two water molecules. Hydrogen bonds shown as the dotted lines between water molecules. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen.
Solved 11 Figure 23 illustrates five water molecules held
What does the dotted line between the water molecules represent? In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. Each water molecule is hydrogen bonded to four. A polar bond a covalent bond a. Hydrogen bonds shown as the dotted lines between water molecules.
SOLVED 17) Based on your knowledge of the polarity of water molecules
The diagram shows two water molecules. A polar bond a covalent bond a. Hydrogen bonds shown as the dotted lines between water molecules. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. This type of bond is a special.
Hydrogen bonding Hopinno
In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. What does the dotted line between the water molecules represent? The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. This type of bond is a special. Each water molecule is hydrogen bonded to four.
The strong polar bond between water molecules creates water cohesion.
Each water molecule is hydrogen bonded to four. A polar bond a covalent bond a. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. The diagram shows two water molecules. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond.
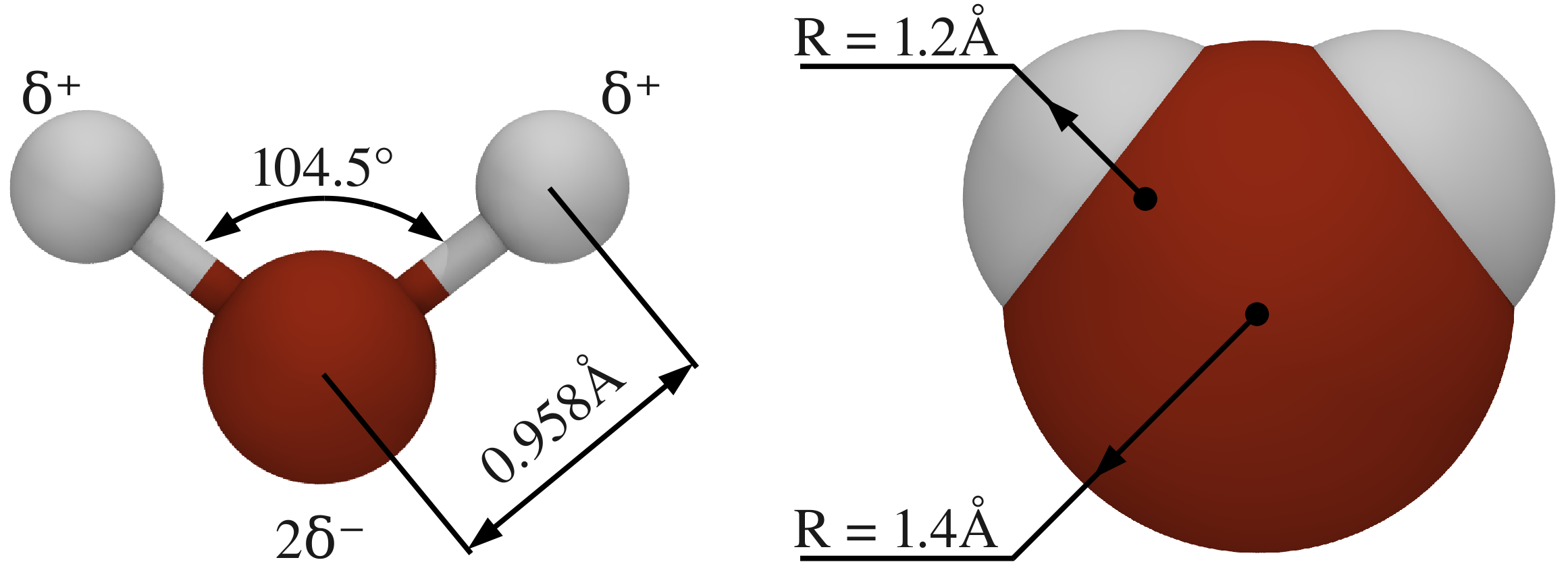
Water molecule — MD simulations documentation
The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. The force of attraction, shown here as a dotted line, is called a hydrogen bond. In the case of water, hydrogen bonds form between. This type of bond is a special. Each water molecule is hydrogen bonded to four.
Nature up close Water molecules CBS News
In the case of water, hydrogen bonds form between. Each water molecule is hydrogen bonded to four. The diagram shows two water molecules. This type of bond is a special. What does the dotted line between the water molecules represent?
Solved The diagram shows two water molecules. What does the dotted
A polar bond a covalent bond a. The force of attraction, shown here as a dotted line, is called a hydrogen bond. Each water molecule is hydrogen bonded to four. What does the dotted line between the water molecules represent? This type of bond is a special.
Best Of How Is Water Molecule Like A
Each water molecule is hydrogen bonded to four. The diagram shows two water molecules. In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. In the case of water, hydrogen bonds form between. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen.
[Solved] Draw five molecules of water (check out figure 2.10 in your
A polar bond a covalent bond a. The diagram shows two water molecules. What does the dotted line between the water molecules represent? In a molecular diagram, the dotted line between water molecules usually represents a hydrogen bond. Hydrogen bonds shown as the dotted lines between water molecules.
In A Molecular Diagram, The Dotted Line Between Water Molecules Usually Represents A Hydrogen Bond.
The diagram shows two water molecules. The dotted line between water molecules represents a hydrogen bond, which forms between the partially positive hydrogen. Hydrogen bonds shown as the dotted lines between water molecules. A polar bond a covalent bond a.
The Force Of Attraction, Shown Here As A Dotted Line, Is Called A Hydrogen Bond.
What does the dotted line between the water molecules represent? Each water molecule is hydrogen bonded to four. In the case of water, hydrogen bonds form between. This type of bond is a special.