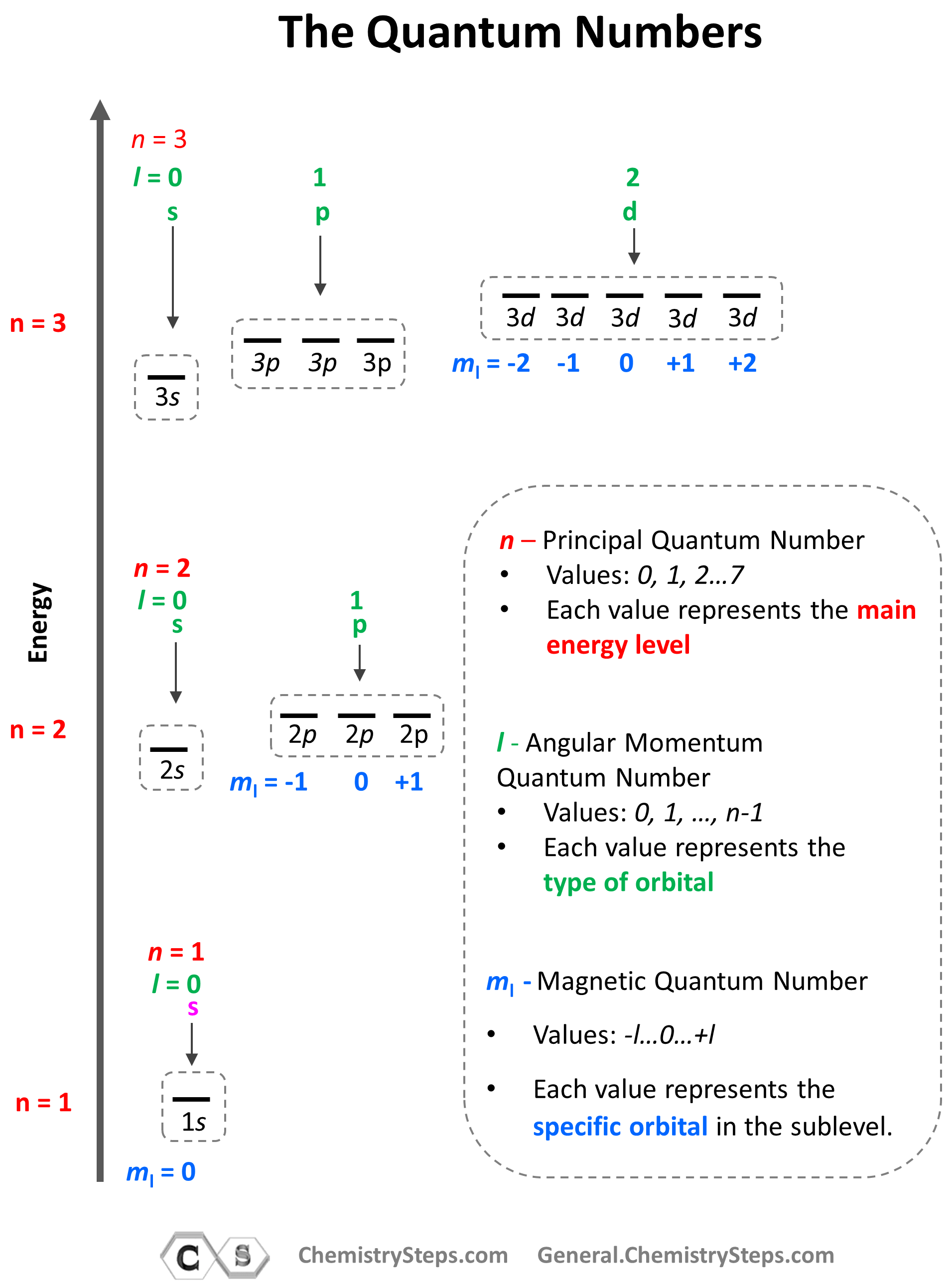
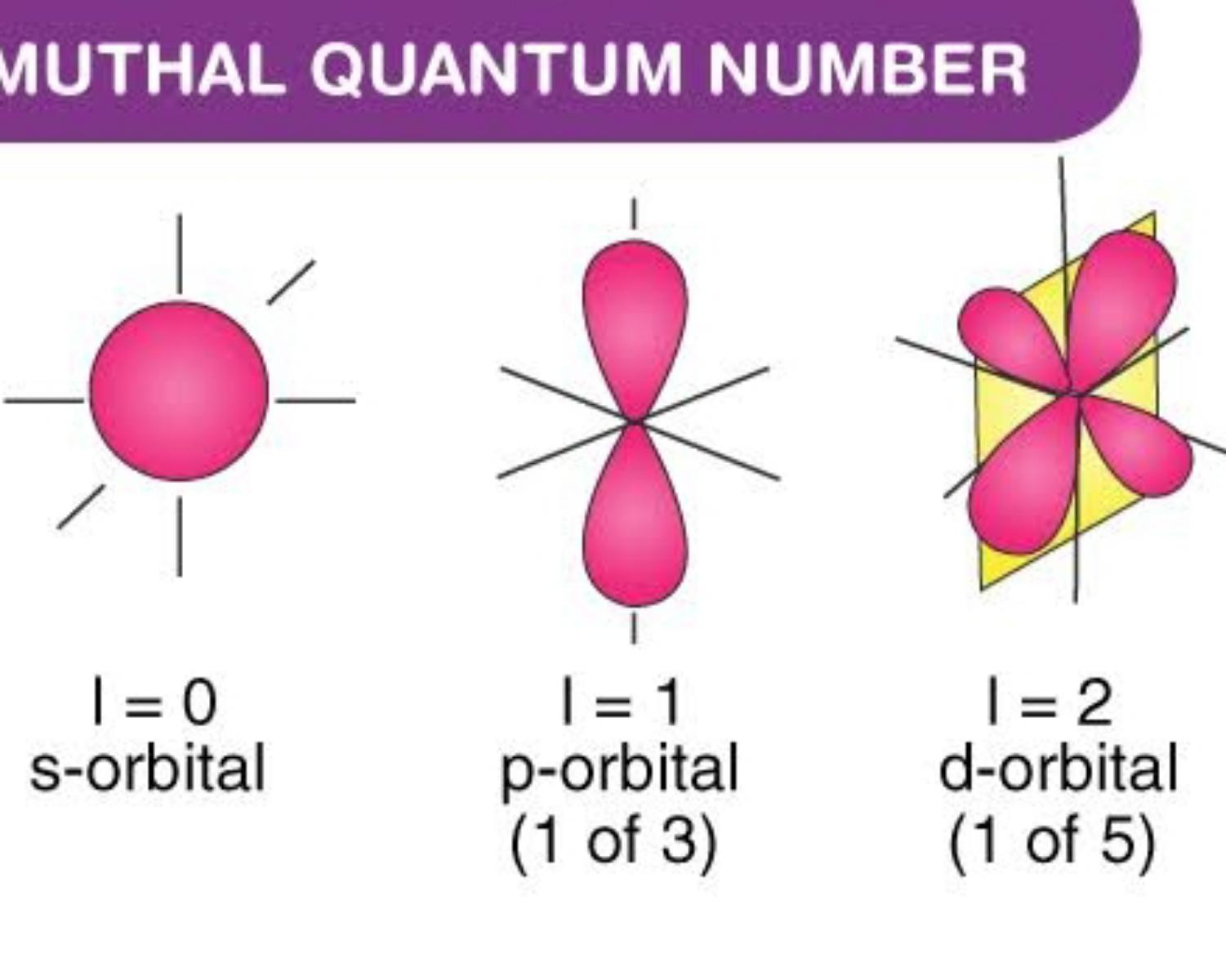
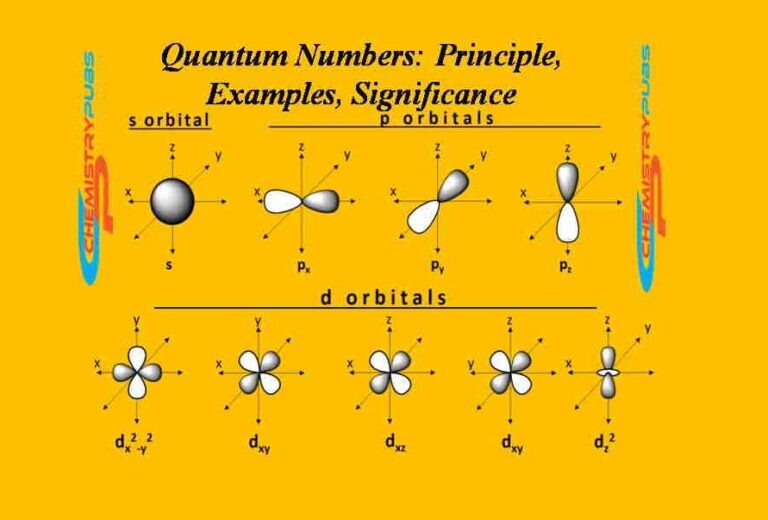
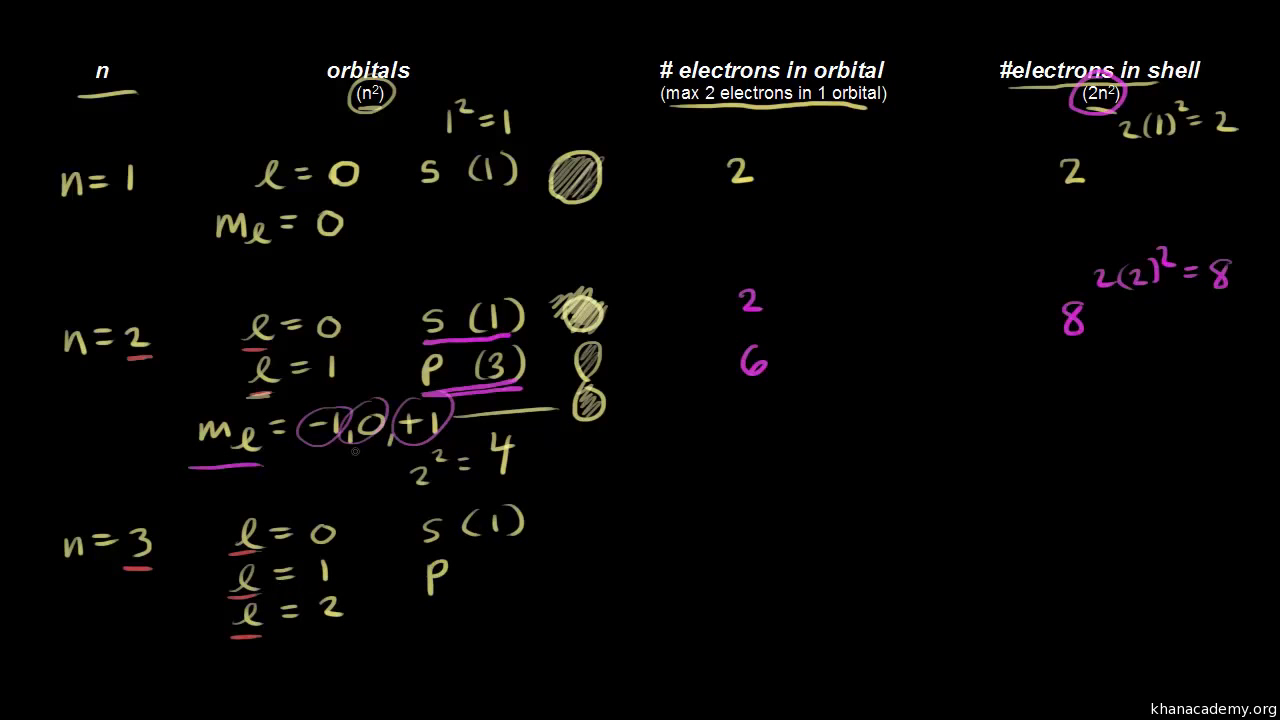
Quantum Numbers Khan Academy
Quantum Numbers Khan Academy - Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. If you're seeing this message, it means we're having trouble loading external resources on our website. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. If you're behind a web filter, please. Learn how quantum numbers are. Explains that only two electrons. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4.
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Learn how quantum numbers are. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. If you're behind a web filter, please. Explains that only two electrons. Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. If you're seeing this message, it means we're having trouble loading external resources on our website.
Explains that only two electrons. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4.
The quantum mechanical model of the atom Quantum numbers and orbitals
Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. If you're behind a web filter, please. Explains that only two electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are.
Quantum numbers Match up
Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Learn how quantum numbers are. If you're seeing this message, it means we're having trouble loading external resources on our website. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Different.
Quantum Numbers Diagram
Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Explains that only two electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons..
Quantum Numbers Section ppt download
Explains that only two electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Learn how quantum numbers are. If you're behind a web filter, please. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons.
Quirky Quantum Fields Khan Academy Breakthrough Junior Challenge 2020
Learn how quantum numbers are. If you're seeing this message, it means we're having trouble loading external resources on our website. Explains that only two electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers.
Electron configurations for the first period Chemistry Khan Academy
Learn how quantum numbers are. Learn how quantum numbers are. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Definition of orbital as region of high probability for finding electron, and how quantum.
SOLUTION Quantum numbers Studypool
In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Explains that only two electrons. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Learn how quantum numbers are. If you're seeing this message, it means we're having trouble loading external.
Spin Quantum Numbers (ms) Deepstash
Learn how quantum numbers are. Explains that only two electrons. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Learn how quantum numbers are.
Quantum Numbers Principle, Examples, Significance Chemistrupubs
Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. If you're.
How To Write Quantum Numbers For Elements
Learn how quantum numbers are. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to.
If You're Seeing This Message, It Means We're Having Trouble Loading External Resources On Our Website.
Explains that only two electrons. Definition of orbital as region of high probability for finding electron, and how quantum numbers are used to describe the orbitals. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. Calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4.
Learn How Quantum Numbers Are.
Learn how quantum numbers are. If you're behind a web filter, please. Different types of orbitals (s, p, d, f) have different shapes and can hold different numbers of electrons. In quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system.