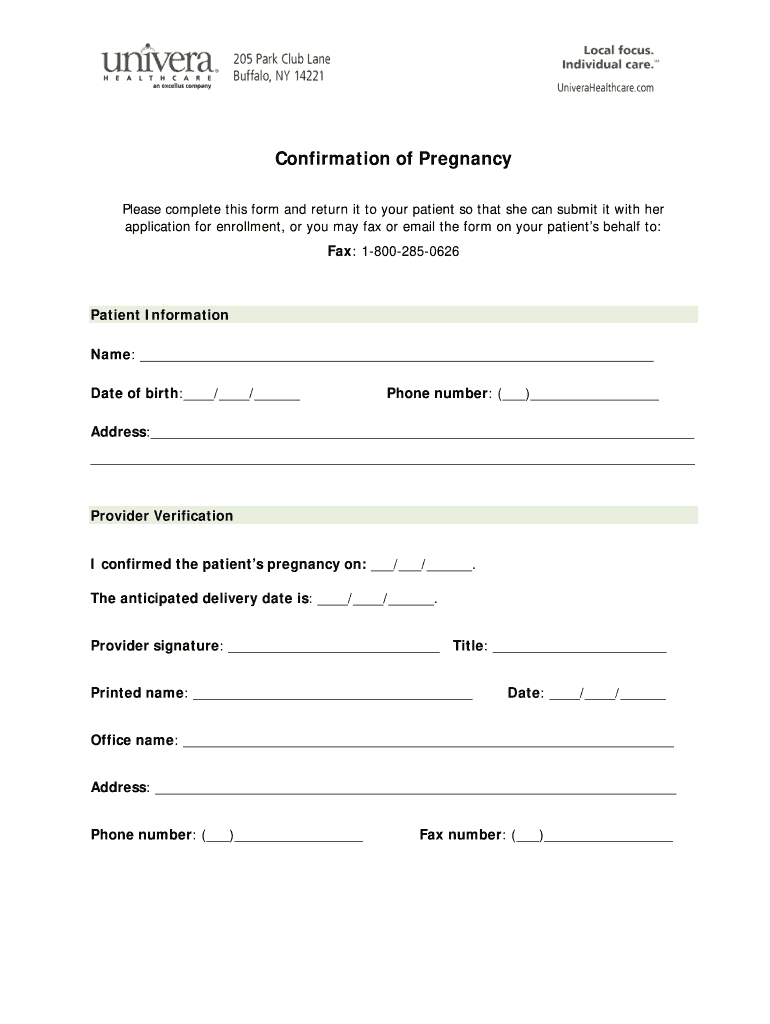
Pregnancy Report Form
Pregnancy Report Form - This form must be used to report any pregnancy in a clinical trial participant. This form must also be used for lactation exposure. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. One pregnancy form should be filled out as an initial. Pregnancy information date of 1st day of last menstrual.
This form must also be used for lactation exposure. One pregnancy form should be filled out as an initial. Pregnancy information date of 1st day of last menstrual. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. This form must be used to report any pregnancy in a clinical trial participant.
This form must be used to report any pregnancy in a clinical trial participant. One pregnancy form should be filled out as an initial. Pregnancy information date of 1st day of last menstrual. This form must also be used for lactation exposure. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or.
16 Free Pregnancy Verification Forms (Word PDF)
This form must also be used for lactation exposure. This form must be used to report any pregnancy in a clinical trial participant. Pregnancy information date of 1st day of last menstrual. One pregnancy form should be filled out as an initial. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame.
Ask a Gynecologist Online for Pregnancy Report Suggestions
One pregnancy form should be filled out as an initial. This form must also be used for lactation exposure. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. Pregnancy information date of 1st day of last menstrual. This form must be used to report any pregnancy in a.
Pregnancy Report Pdf 20202022 Fill and Sign Printable Template
The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. This form must also be used for lactation exposure. Pregnancy information date of 1st day of last menstrual. This form must be used to report any pregnancy in a clinical trial participant. One pregnancy form should be filled out.
Pregnancy Consent Form Printable Consent Form
The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. This form must be used to report any pregnancy in a clinical trial participant. One pregnancy form should be filled out as an initial. Pregnancy information date of 1st day of last menstrual. This form must also be used.
Pregnancy Verification Letter Edit & Share airSlate SignNow
Pregnancy information date of 1st day of last menstrual. This form must also be used for lactation exposure. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. This form must be used to report any pregnancy in a clinical trial participant. One pregnancy form should be filled out.
Hospital Positive Pregnancy Paperwork 20142024 Form Fill Out and
This form must be used to report any pregnancy in a clinical trial participant. This form must also be used for lactation exposure. One pregnancy form should be filled out as an initial. Pregnancy information date of 1st day of last menstrual. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame.
Consent 20132024 Form Fill Out and Sign Printable PDF Template
One pregnancy form should be filled out as an initial. This form must also be used for lactation exposure. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. Pregnancy information date of 1st day of last menstrual. This form must be used to report any pregnancy in a.
Pregnancy Results Paper Form Fill Out and Sign Printable PDF Template
The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. One pregnancy form should be filled out as an initial. This form must be used to report any pregnancy in a clinical trial participant. Pregnancy information date of 1st day of last menstrual. This form must also be used.
Pregnancy Health Record Fill and Sign Printable Template Online US
Pregnancy information date of 1st day of last menstrual. One pregnancy form should be filled out as an initial. This form must also be used for lactation exposure. This form must be used to report any pregnancy in a clinical trial participant. The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame.
Pregnancy Notification Report Fill Online, Printable, Fillable, Blank
The pregnancy report form is designed to specifically follow mothers and foetuses/children exposed to drugs in the frame of cts or. This form must be used to report any pregnancy in a clinical trial participant. This form must also be used for lactation exposure. Pregnancy information date of 1st day of last menstrual. One pregnancy form should be filled out.
The Pregnancy Report Form Is Designed To Specifically Follow Mothers And Foetuses/Children Exposed To Drugs In The Frame Of Cts Or.
Pregnancy information date of 1st day of last menstrual. This form must also be used for lactation exposure. This form must be used to report any pregnancy in a clinical trial participant. One pregnancy form should be filled out as an initial.