Predicting Products Of Chemical Reactions Worksheet
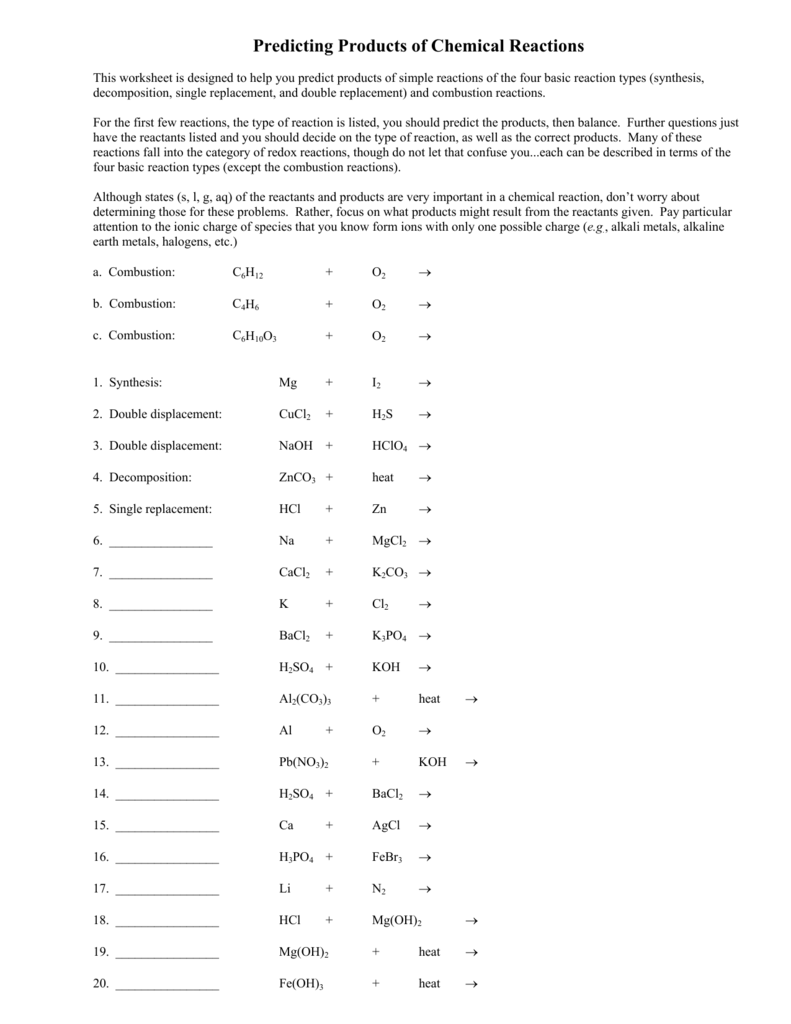
Predicting Products Of Chemical Reactions Worksheet - Predict the products of the reactions below. Predict the products for, and then balance each of the following chemical reactions: Use your activity series, predicting products helper sheet and the solubility rules. Then, write the balanced equation and classify the reaction. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. If a precipitate forms, indicate by circling the precipitate. Sii4 + mg → (single replacement) This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Predict the products of the reactions below.
This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Use your activity series, predicting products helper sheet and the solubility rules. Predict the products of the reactions below. Predict the products for, and then balance each of the following chemical reactions: Sii4 + mg → (single replacement) If a precipitate forms, indicate by circling the precipitate. Predict the products of the reactions below. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Then, write the balanced equation and classify the reaction.
Sii4 + mg → (single replacement) Predict the products of the reactions below. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Use your activity series, predicting products helper sheet and the solubility rules. Predict the products of the reactions below. Predict the products for, and then balance each of the following chemical reactions: This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Then, write the balanced equation and classify the reaction. If a precipitate forms, indicate by circling the precipitate.
30++ Predicting Products Of Chemical Reactions Worksheet Worksheets
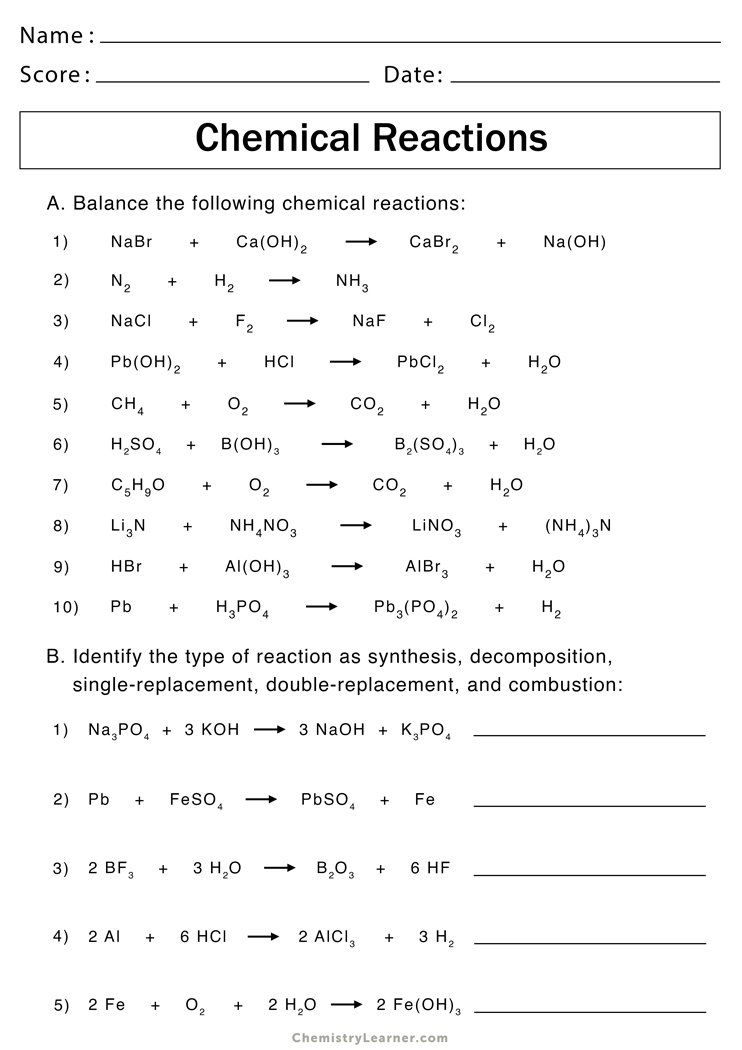
Predict the products of the reactions below. Then, write the balanced equation and classify the reaction. Predict the products of the reactions below. If a precipitate forms, indicate by circling the precipitate. Predict the products for, and then balance each of the following chemical reactions:
30++ Predicting Products Of Chemical Reactions Worksheet Worksheets
Predict the products for, and then balance each of the following chemical reactions: Use your activity series, predicting products helper sheet and the solubility rules. Predict the products of the reactions below. If a precipitate forms, indicate by circling the precipitate. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis,.
Predicting Products Of Reactions Chem Worksheet 10 4 Answer Key — db
Use your activity series, predicting products helper sheet and the solubility rules. Predict the products for, and then balance each of the following chemical reactions: This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Silver nitrate + zinc chloride → zinc nitrate +.
Predicting Products of Chemical Reactions
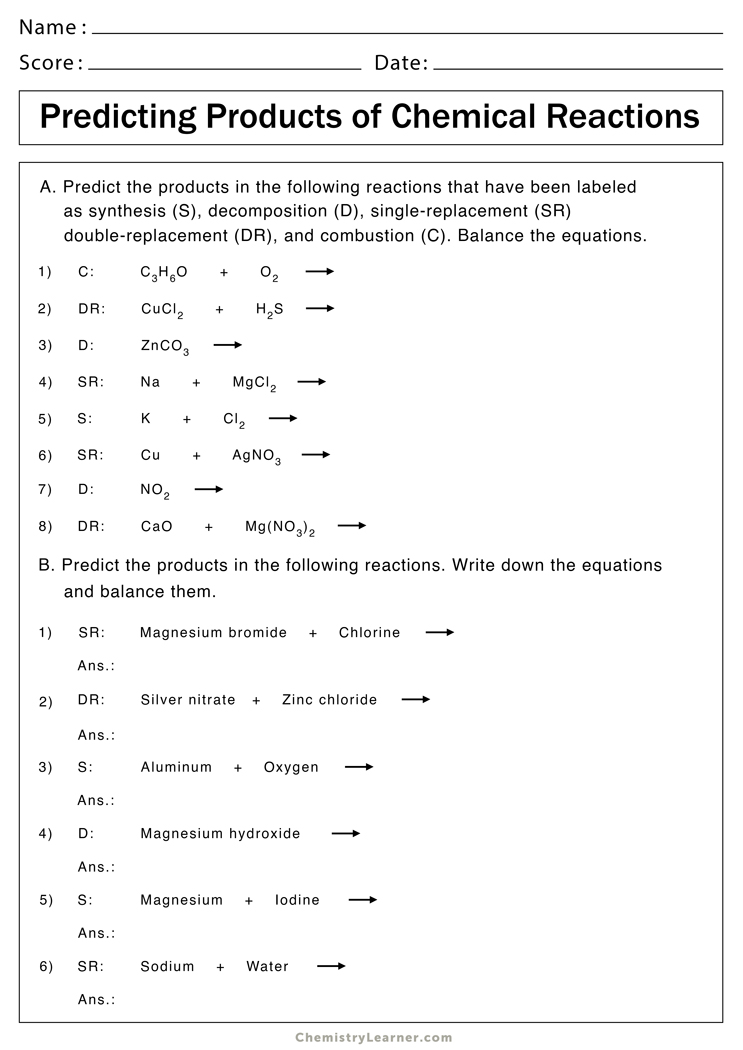
Use your activity series, predicting products helper sheet and the solubility rules. Then, write the balanced equation and classify the reaction. Predict the products of the reactions below. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Silver nitrate + zinc chloride →.
Identifying Reaction Types Worksheet
This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Use your activity series, predicting products helper sheet and the solubility rules. Predict the products for, and then balance each of the following chemical reactions: Predict the products of the reactions below. This worksheet.
30++ Predicting Products Of Chemical Reactions Worksheet Worksheets
If a precipitate forms, indicate by circling the precipitate. Predict the products of the reactions below. Sii4 + mg → (single replacement) This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. This worksheet is designed to help you predict products of simple reactions.
Predicting Products Of Chemical Reactions Worksheet Answers —
Predict the products of the reactions below. If a precipitate forms, indicate by circling the precipitate. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Then, write.
Chemical Reactions Worksheet Answers Predicting —
Silver nitrate + zinc chloride → zinc nitrate + potassium iodide → iron iii chloride +. Predict the products for, and then balance each of the following chemical reactions: Sii4 + mg → (single replacement) This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement).
Predicting The Products Of Chemical Reactions Worksheets
Use your activity series, predicting products helper sheet and the solubility rules. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Predict the products of the reactions below. If a precipitate forms, indicate by circling the precipitate. Then, write the balanced equation and.
Types of Chemical Reactions Worksheets Free Printable
Use your activity series, predicting products helper sheet and the solubility rules. Sii4 + mg → (single replacement) If a precipitate forms, indicate by circling the precipitate. Predict the products of the reactions below. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and.
Silver Nitrate + Zinc Chloride → Zinc Nitrate + Potassium Iodide → Iron Iii Chloride +.
Predict the products of the reactions below. Use your activity series, predicting products helper sheet and the solubility rules. If a precipitate forms, indicate by circling the precipitate. This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and.
Predict The Products Of The Reactions Below.
Predict the products for, and then balance each of the following chemical reactions: This worksheet is designed to help you predict products of simple reactions of the four basic reaction types (synthesis, decomposition, single replacement, and double replacement) and. Then, write the balanced equation and classify the reaction. Sii4 + mg → (single replacement)