Most Reduced Form Of Carbon
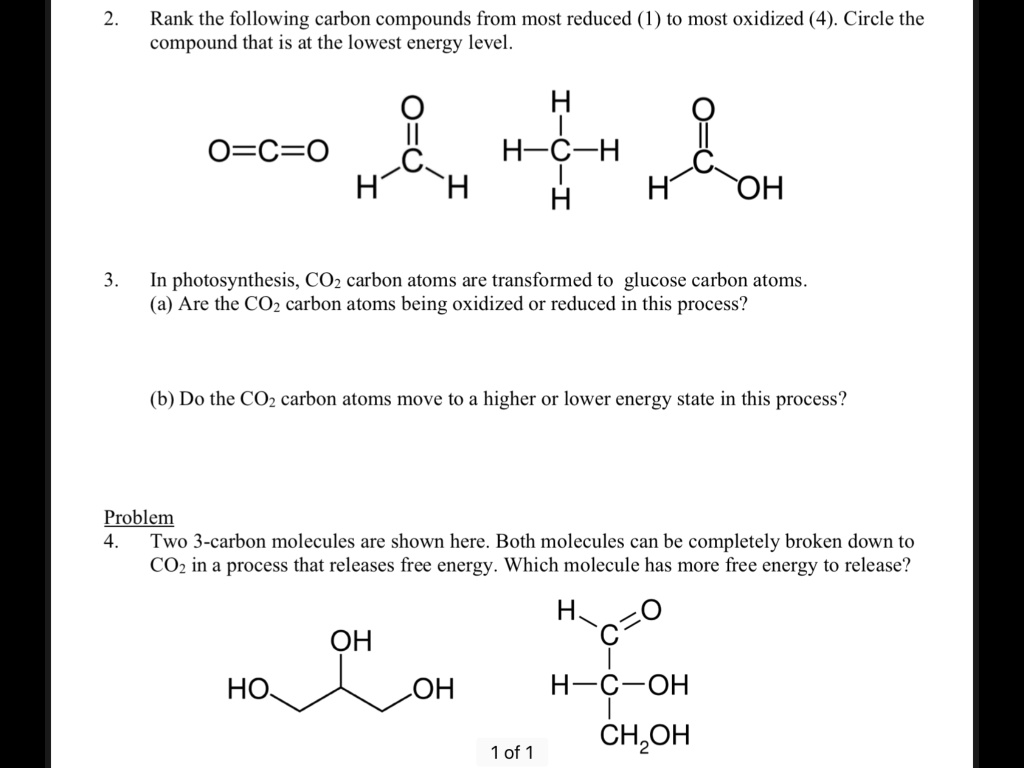
Most Reduced Form Of Carbon - Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most oxidized form of carbon? This is because, in methane, each carbon atom forms. Which of the following is the most reduced form of carbon? Ketones and aldehydes can be reduced with h2 & ni, pd, or pt, although it is much more common to use lialh or nabh for the same reduction. For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. Which molecule represents the most reduced form of carbon? A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol (ch3oh) In the given options, methane (ch4) is the most reduced form of carbon. For example, ch4 has the most reduced form of. When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative).
Which of the following is the most reduced form of carbon? This is because, in methane, each carbon atom forms. For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative). Which molecule represents the most reduced form of carbon? A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol (ch3oh) Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most oxidized form of carbon? For example, ch4 has the most reduced form of. Study with quizlet and memorize flashcards containing terms like 1. In the given options, methane (ch4) is the most reduced form of carbon.
This is because, in methane, each carbon atom forms. In the given options, methane (ch4) is the most reduced form of carbon. Ketones and aldehydes can be reduced with h2 & ni, pd, or pt, although it is much more common to use lialh or nabh for the same reduction. For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative). Study with quizlet and memorize flashcards containing terms like 1. For example, ch4 has the most reduced form of. Which molecule represents the most reduced form of carbon? Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most oxidized form of carbon? Which of the following is the most reduced form of carbon?
What is carbon capture and how does it work? The Australian
For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative). This is because, in methane, each carbon atom forms. Study with quizlet and memorize flashcards containing terms like 1. Which molecule represents.
SOLVED Rank the following carbon compounds from most reduced to most
Which molecule represents the most reduced form of carbon? Ketones and aldehydes can be reduced with h2 & ni, pd, or pt, although it is much more common to use lialh or nabh for the same reduction. A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol (ch3oh) Study with quizlet and memorize flashcards containing terms like 1. When the.
What is the carbon cycle? Doc Template pdfFiller
Study with quizlet and memorize flashcards containing terms like 1. In the given options, methane (ch4) is the most reduced form of carbon. Ketones and aldehydes can be reduced with h2 & ni, pd, or pt, although it is much more common to use lialh or nabh for the same reduction. When the oxidizing agent is reduced, its oxidation state.
Low Carbon Living Calculator
For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. For example, ch4 has the most reduced form of. Ketones and aldehydes can be reduced with h2 & ni, pd, or pt, although it is much more common to use lialh or nabh for the.
Carbon 101 — Carbon Yield
A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol (ch3oh) In the given options, methane (ch4) is the most reduced form of carbon. For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. Study with quizlet and memorize flashcards containing terms like oxidation state,.
CBSE X Chemistry Carbon and its compounds Forms of Carbon YouTube
For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol (ch3oh) Which molecule represents the most reduced form of carbon? This is because, in methane, each carbon atom forms. For example, ch4 has the most.
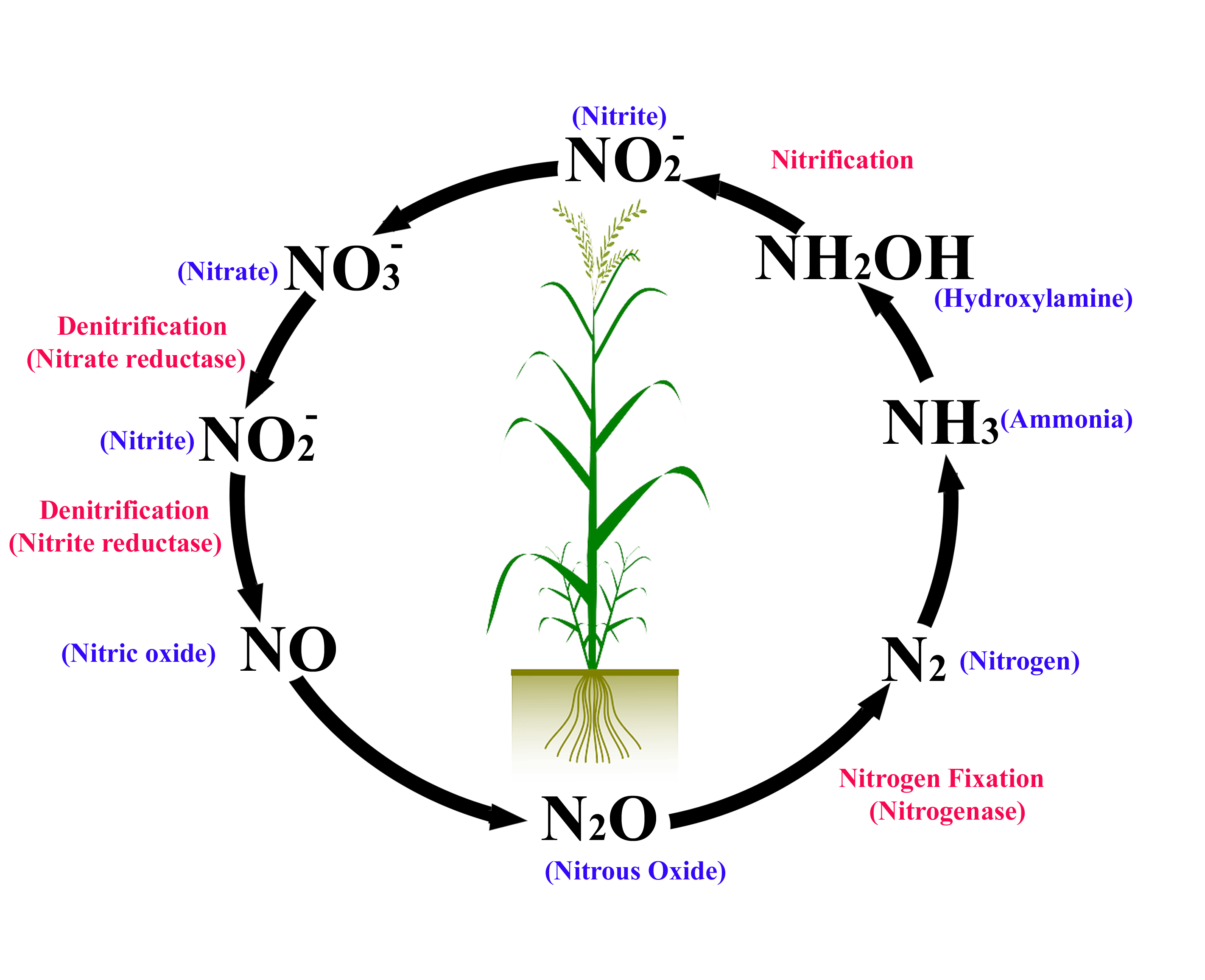
Role of Nitrogen in Crops BioChemiThon BioChemiThon
For example, ch4 has the most reduced form of. Study with quizlet and memorize flashcards containing terms like 1. In the given options, methane (ch4) is the most reduced form of carbon. When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative). Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most.
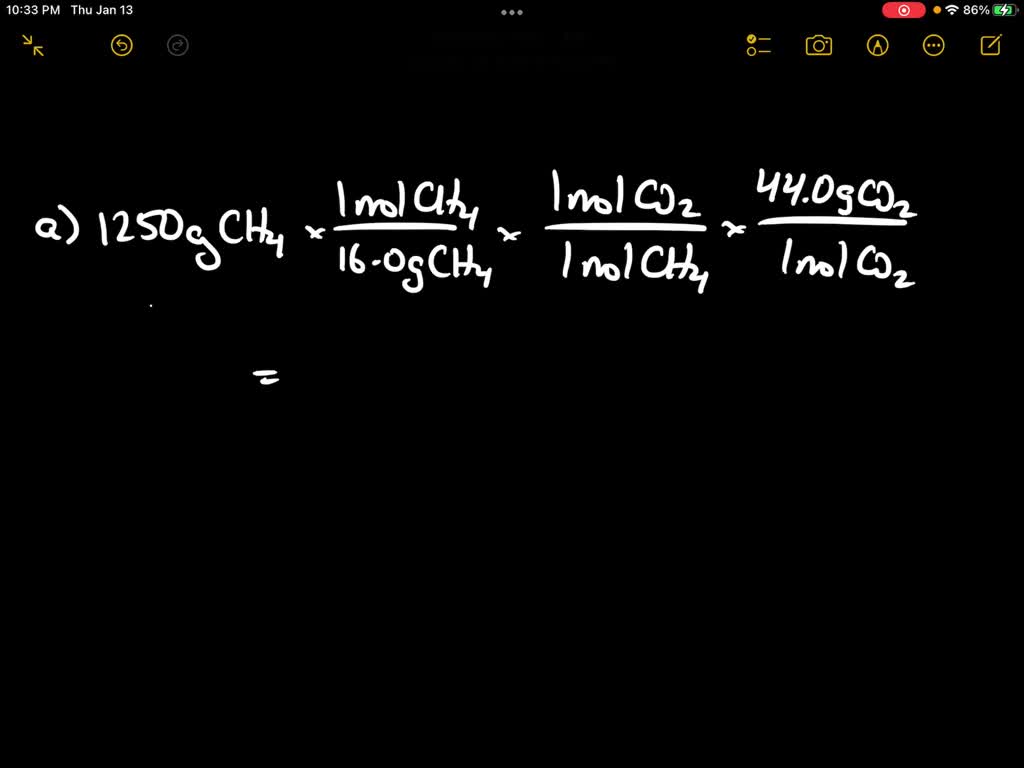
SOLVED Methane (CH4, 16.05 g/mol) reacts with oxygen to form carbon
For example, ch4 has the most reduced form of. Which molecule represents the most reduced form of carbon? Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most oxidized form of carbon? When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative). A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol.
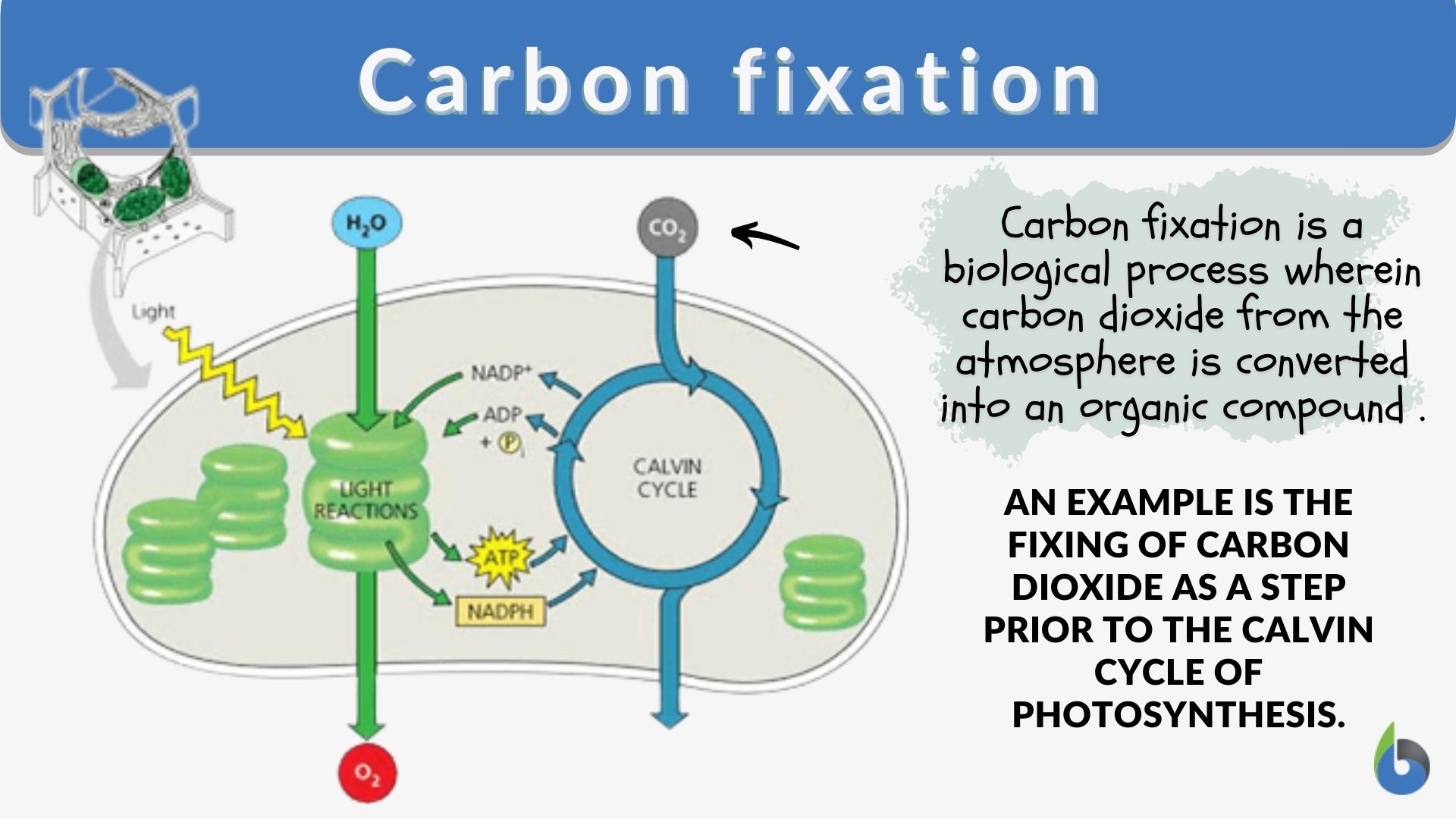
Carbon fixation Definition and Examples Biology Online Dictionary
For example, ch4 has the most reduced form of. Which molecule represents the most reduced form of carbon? Which of the following is the most reduced form of carbon? For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon. This is because, in methane, each.
Carbon County Economic Development Corporation
For example, ch4 has the most reduced form of. Which of the following is the most reduced form of carbon? A.carbon dioxide (co2) b.formic acid ( hcooh) c.methane (ch4) d.methanol (ch3oh) Study with quizlet and memorize flashcards containing terms like 1. Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most oxidized form of carbon?
In The Given Options, Methane (Ch4) Is The Most Reduced Form Of Carbon.
This is because, in methane, each carbon atom forms. Which of the following is the most reduced form of carbon? Study with quizlet and memorize flashcards containing terms like oxidation state, what is the most oxidized form of carbon? For organic compounds, it helps to remember that the most reduced form of carbon is methane and the most oxidized form of carbon is carbon.
A.carbon Dioxide (Co2) B.formic Acid ( Hcooh) C.methane (Ch4) D.methanol (Ch3Oh)
Ketones and aldehydes can be reduced with h2 & ni, pd, or pt, although it is much more common to use lialh or nabh for the same reduction. When the oxidizing agent is reduced, its oxidation state decreases (becomes more negative). Which molecule represents the most reduced form of carbon? For example, ch4 has the most reduced form of.