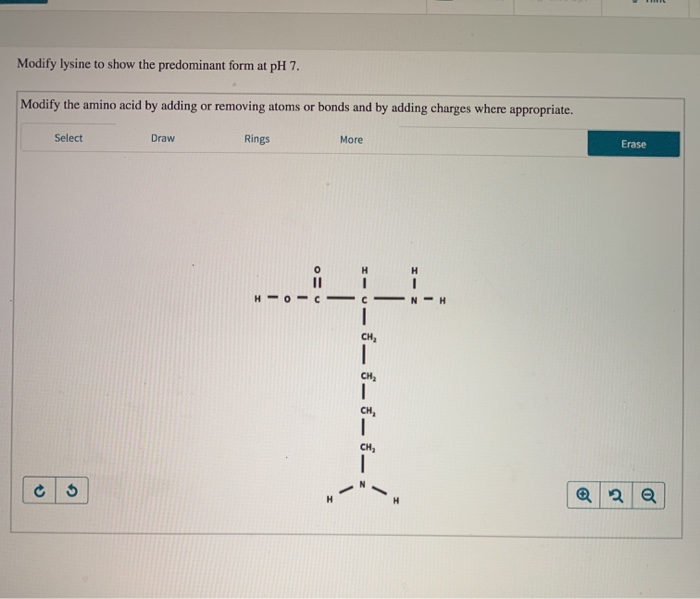
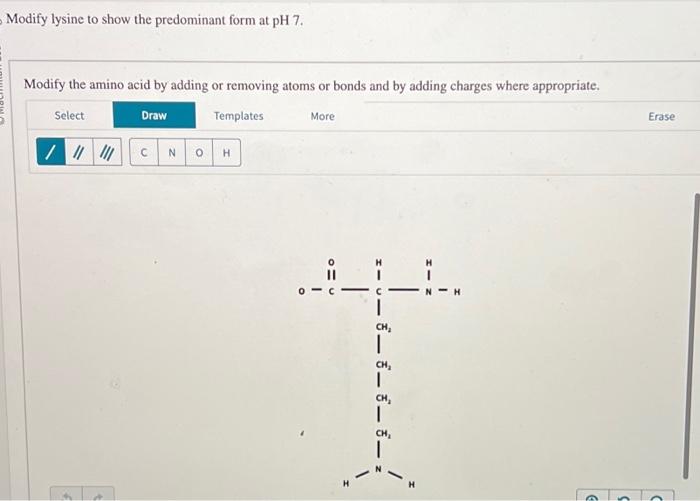
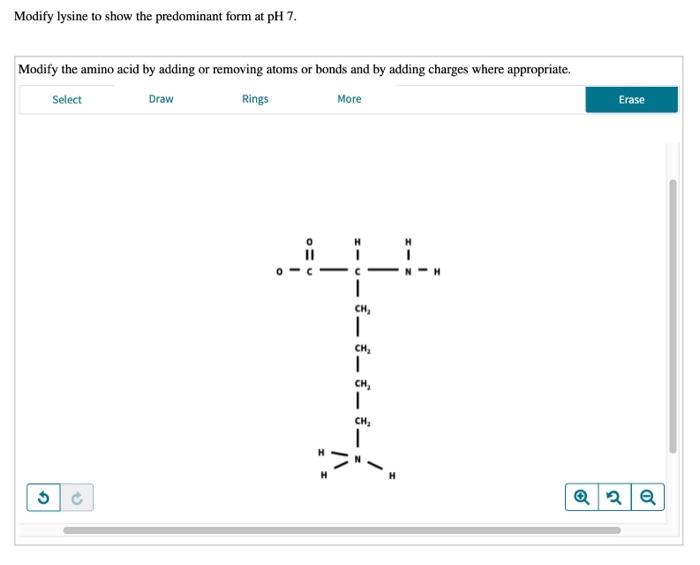
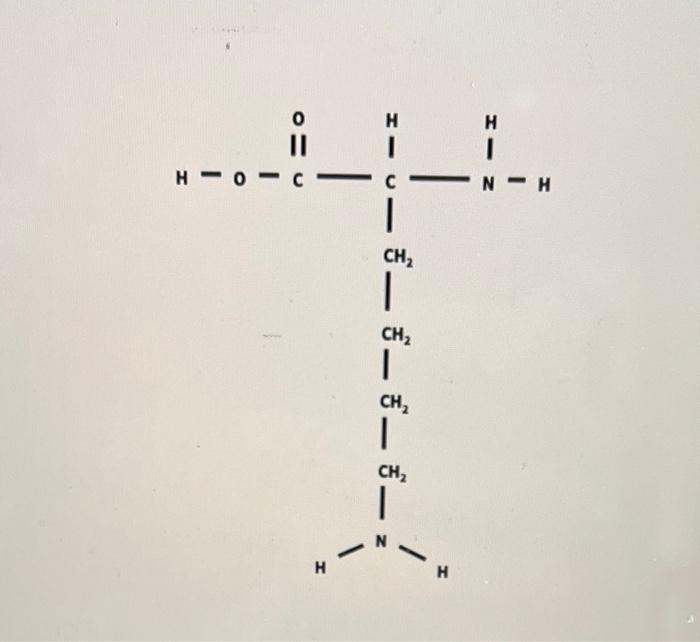
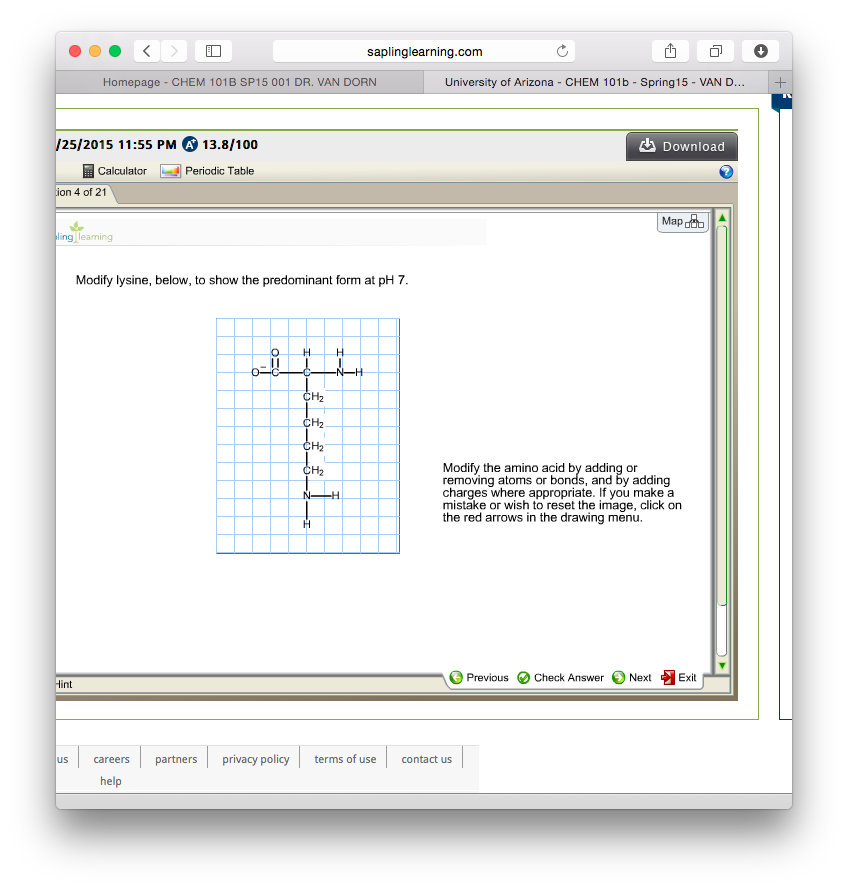
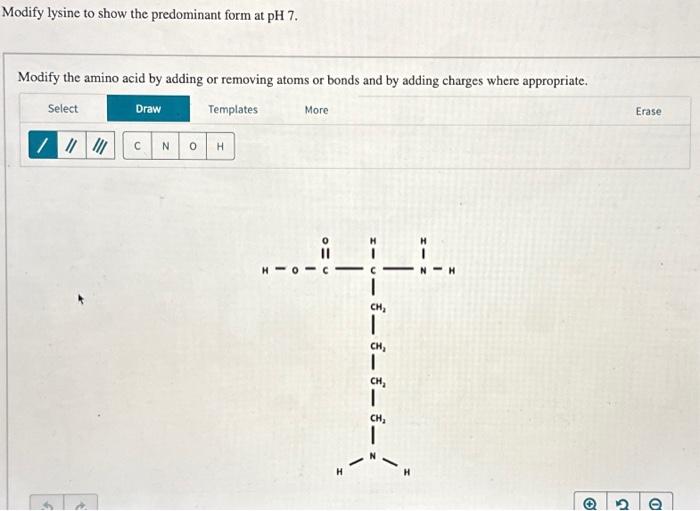
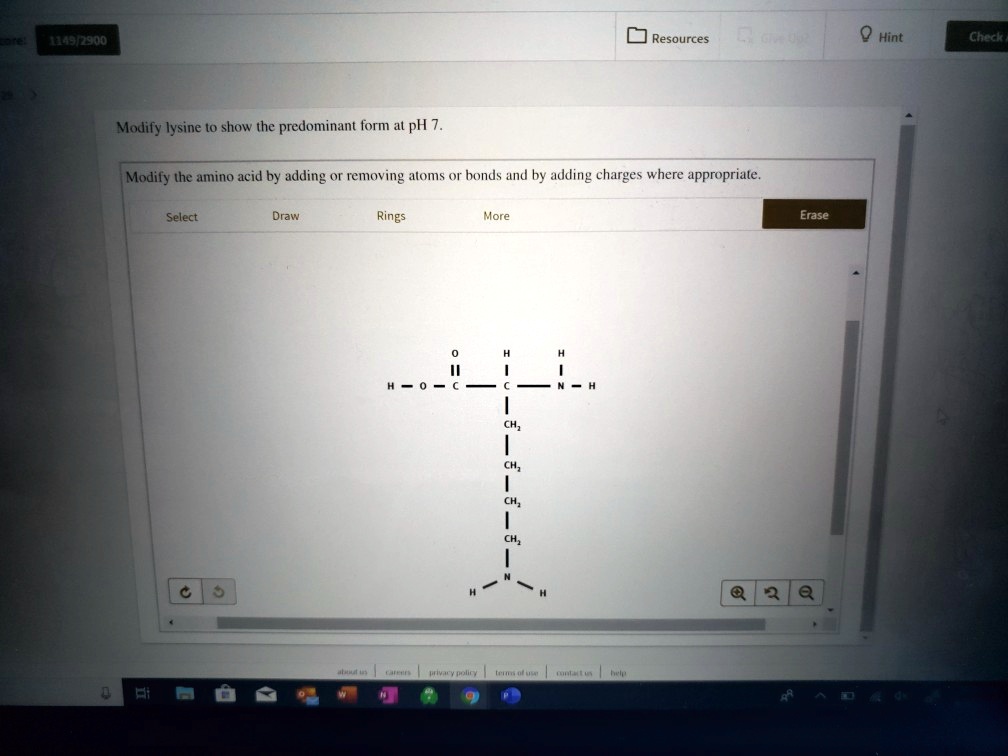
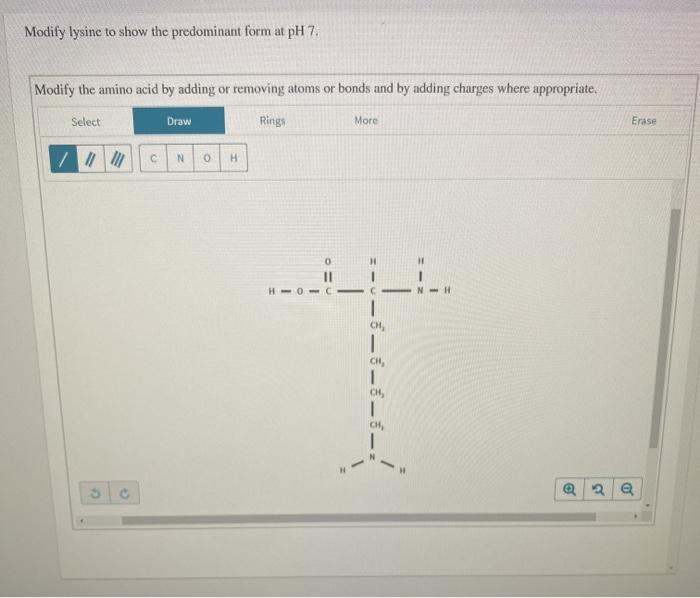
Modify Lysine To Show The Predominant Form At Ph 7
Modify Lysine To Show The Predominant Form At Ph 7 - The ph 7, suggests neutral state, that is, no proton in the solution. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Its composition is given in the attachment below. Modify the amino acid by adding or. Removing atoms or bonds and by adding charges where appropriate. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine.
To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. The ph 7, suggests neutral state, that is, no proton in the solution. Removing atoms or bonds and by adding charges where appropriate. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Modify the amino acid by adding or. Its composition is given in the attachment below.
Its composition is given in the attachment below. The ph 7, suggests neutral state, that is, no proton in the solution. Removing atoms or bonds and by adding charges where appropriate. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Modify the amino acid by adding or. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications.
Solved Modify lysine to show the predominant form at pH 7.
The ph 7, suggests neutral state, that is, no proton in the solution. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Its composition is given in the attachment below. Modify the amino acid by adding or. Removing atoms or bonds and by adding charges.
Solved Modify lysine to show the predominant form at pH7.
To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. The ph 7, suggests neutral state, that is, no proton in the solution. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Its composition is given.
Modify lysine, below, to show the predominant form at pH 7. Quizlet
This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Modify the amino acid by adding or. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Removing atoms or bonds and by adding charges where appropriate..
Solved Modify lysine to show the predominant form at pH 7.
The ph 7, suggests neutral state, that is, no proton in the solution. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Removing atoms or bonds.
Solved modify lysine to show the predominant form at pH 7
The ph 7, suggests neutral state, that is, no proton in the solution. Removing atoms or bonds and by adding charges where appropriate. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Modify the amino acid by adding or. This article explores the protonation and.
Modify lysine, below, to show the predominant form at
Modify the amino acid by adding or. The ph 7, suggests neutral state, that is, no proton in the solution. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Removing atoms or bonds and by adding charges where appropriate. This article explores the protonation and.
Solved Modify lysine to show the predominant form at pH7.
This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Modify the amino acid by adding or. Its composition is given in the attachment below. The ph.
SOLVED aatsno Resources Hint Check Modify lysine to show the
Modify the amino acid by adding or. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Removing atoms or bonds and by adding charges where appropriate. The ph 7, suggests neutral state, that is, no proton in the solution. Its composition is given in the attachment below.
Solved Modify lysine to show the predominant form at pH 7.
Its composition is given in the attachment below. Removing atoms or bonds and by adding charges where appropriate. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. The ph 7, suggests neutral state, that is, no proton in the solution. Modify the amino acid by.
Lysine Structure At Different Ph
To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Its composition is given in the attachment below. Modify the amino acid by adding or. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. The ph.
To Determine The Predominant Form Of Lysine At Ph 7, We Need To Consider The Pka Values Of The Functional Groups Present In Lysine.
The ph 7, suggests neutral state, that is, no proton in the solution. This article explores the protonation and deprotonation of lysine, identifying the predominant form at ph 7 and its implications. Removing atoms or bonds and by adding charges where appropriate. Modify the amino acid by adding or.