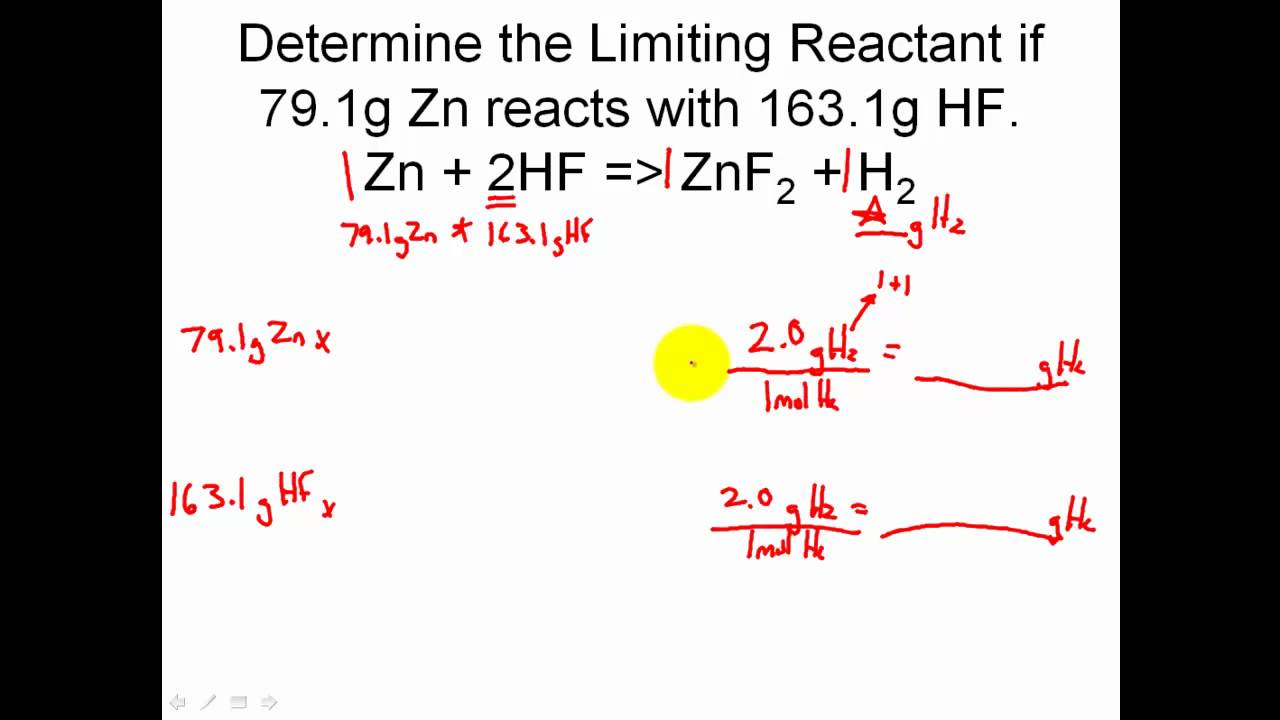
Limiting Reactant Khan Academy
Limiting Reactant Khan Academy - Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with 4 molecules of. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from a limiting reactant
Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. A) 3 atoms of carbon combine with 4 molecules of. Calculating the amount of product formed from a limiting reactant Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions.
A) 3 atoms of carbon combine with 4 molecules of. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from a limiting reactant Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant.
how to solve limiting reactant
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from a limiting reactant Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with.
Limiting reactant (example problem) Chemistry Khan Academy Urdu
Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from a limiting reactant Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them.
Limiting Reactants. ppt download
A) 3 atoms of carbon combine with 4 molecules of. Calculating the amount of product formed from a limiting reactant Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Determine limiting and excess reagent and the amount of unreacted excess reactant. Learn about limiting reactants, reaction yields, and how to calculate them.
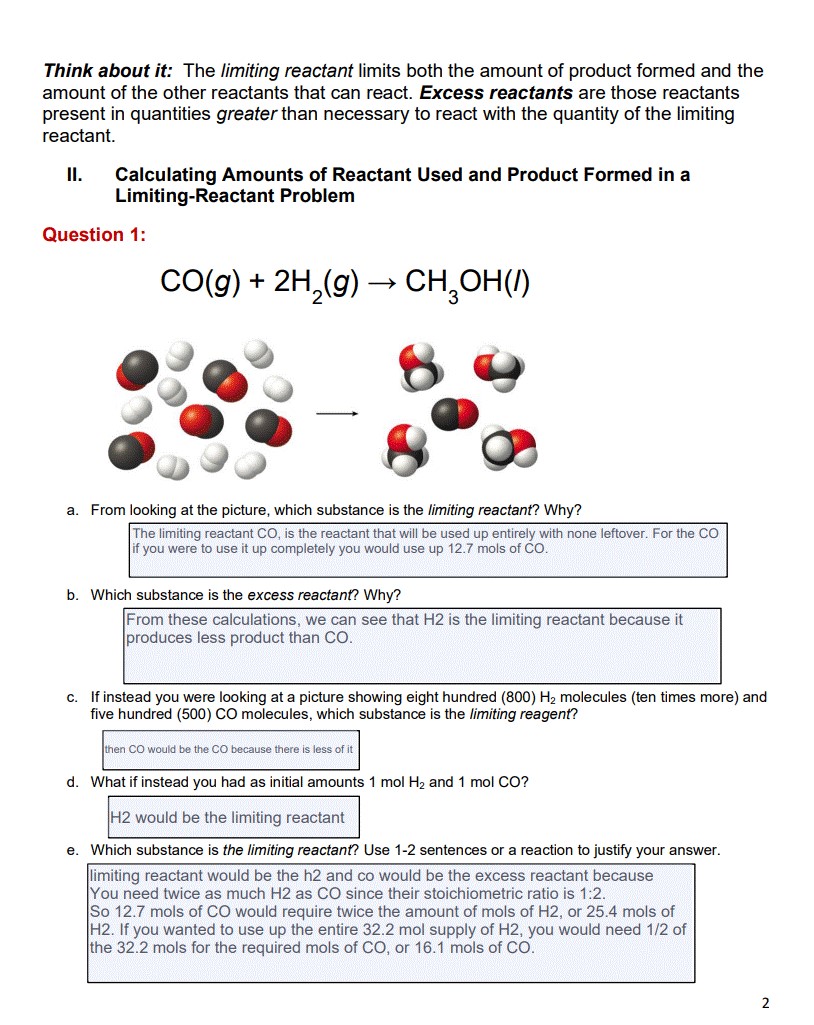
Solved Think about it The limiting reactant limits both the
A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant Limiting reactant example problem here’s a complex example problem with a limiting reactant worked.
Limiting Reactant Worksheet Answers Englishworksheet.my.id
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from a limiting reactant A) 3 atoms of carbon combine with 4 molecules of. Determine limiting and excess reagent and the amount of.
Limiting reactant example problem 1 Chemistry Khan Academy YouTube
A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from a limiting reactant Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked.
Limiting reactant example problem 1 Chemistry Khan Academy (With
Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant A) 3 atoms of carbon combine with 4 molecules of. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them.
Limiting Reactant Example Worksheet
Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. A) 3 atoms of carbon combine with.
Limiting Reactants GCSE Lesson (SC9c CC9c) Teaching Resources
Calculating the amount of product formed from a limiting reactant A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked.
SOLUTION Limiting reactant detailed topic Studypool
Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. A) 3 atoms of carbon combine with.
Determine Limiting And Excess Reagent And The Amount Of Unreacted Excess Reactant.
Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from a limiting reactant A) 3 atoms of carbon combine with 4 molecules of.