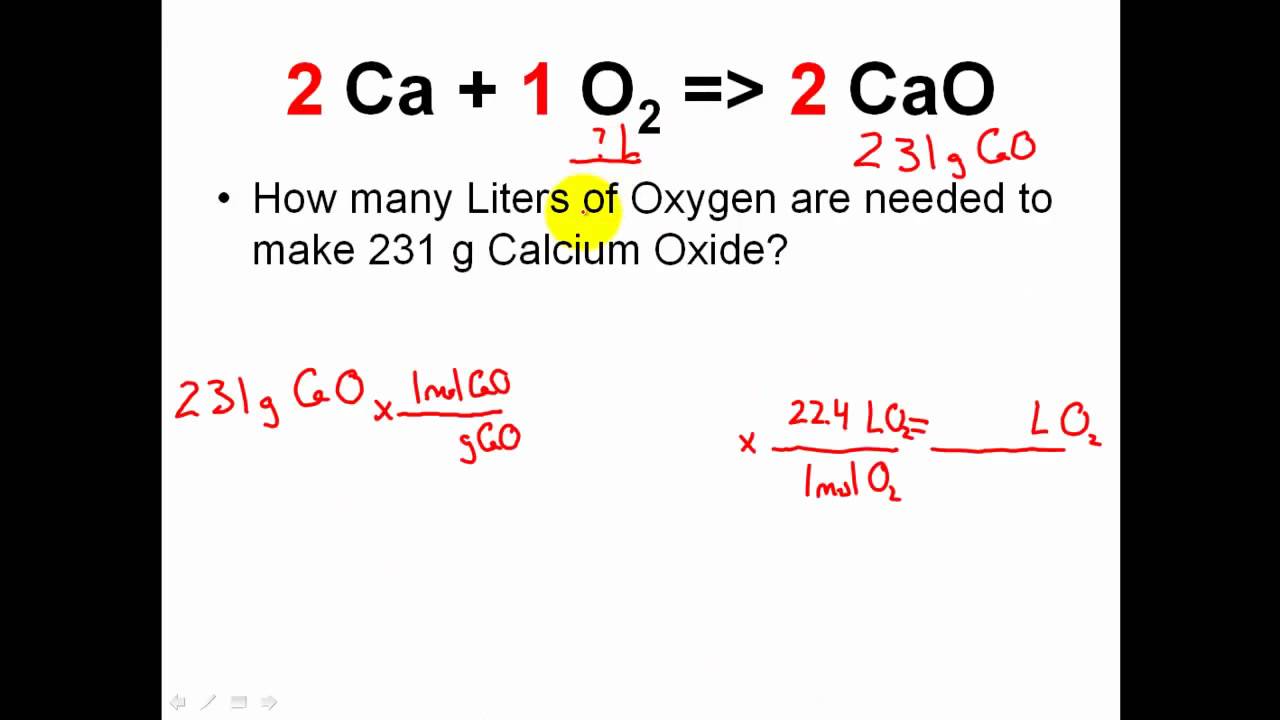
Khan Academy Stoichiometry
Khan Academy Stoichiometry - Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. We'll get right to the point: Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please.
We'll get right to the point: We're asking you to help support khan academy. We're a nonprofit that relies on support from people like you. Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website.
We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. We'll get right to the point: Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another.
Balancing And Stoichiometry Worksheet Balancing Chemical Equations 1
If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website. We'll get right to the point: We're asking you to help support khan academy. Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another.
Khan Academy Chemistry Introduction to Stoichiometry Instructional
We're a nonprofit that relies on support from people like you. We're asking you to help support khan academy. Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. If you're behind a web filter, please. We'll get right to the point:
Khan Academy Chemistry Stoichiometry Limiting Reagent Instructional
If you're behind a web filter, please. We're asking you to help support khan academy. Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. We'll get right to the point: If you're seeing this message, it means we're having trouble loading external resources on our website.
Stoichiometry example problem 1 Chemistry Khan Academy YouTube
We'll get right to the point: Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. We're asking you to help support khan academy. If you're seeing this message, it means we're having trouble loading external resources on our website. We're a nonprofit that relies on support from people like you.
Stoichiometry Chemical reactions and stoichiometry Chemistry Khan
We'll get right to the point: We're asking you to help support khan academy. Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website.
Stoichiometry example problem 1 Chemistry Khan Academy Chemistry
We're asking you to help support khan academy. Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. We're a nonprofit that relies on support from people like you. If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website.
Khan Academy Stoichiometry and the Properties of Acetic Acid Unknown
Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. We're asking you to help support khan academy. Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. We're a nonprofit that relies on support from people like you. If you're seeing this message, it.
Stoichiometry example problem 2 Chemistry Khan Academy YouTube
Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. We'll get right to the point: We're asking you to help support khan academy. If you're behind a web filter, please. We're a nonprofit that relies on support from people like you.
Chapter 12 Stoichiometry
If you're seeing this message, it means we're having trouble loading external resources on our website. Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. We'll get right to the point: We're a nonprofit that relies on support from people like you. If you're behind a web filter, please.
What Is The Law Of Stoichiometry
Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent. We'll get right to the point: We're asking you to help support khan academy. If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website.
We'll Get Right To The Point:
Stoichiometry, in chemistry, the determination of the proportions in which elements or compounds react with one another. We're asking you to help support khan academy. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please.
We're A Nonprofit That Relies On Support From People Like You.
Discover how to use balanced chemical equations to convert between moles of reactants and products, and calculate percent.