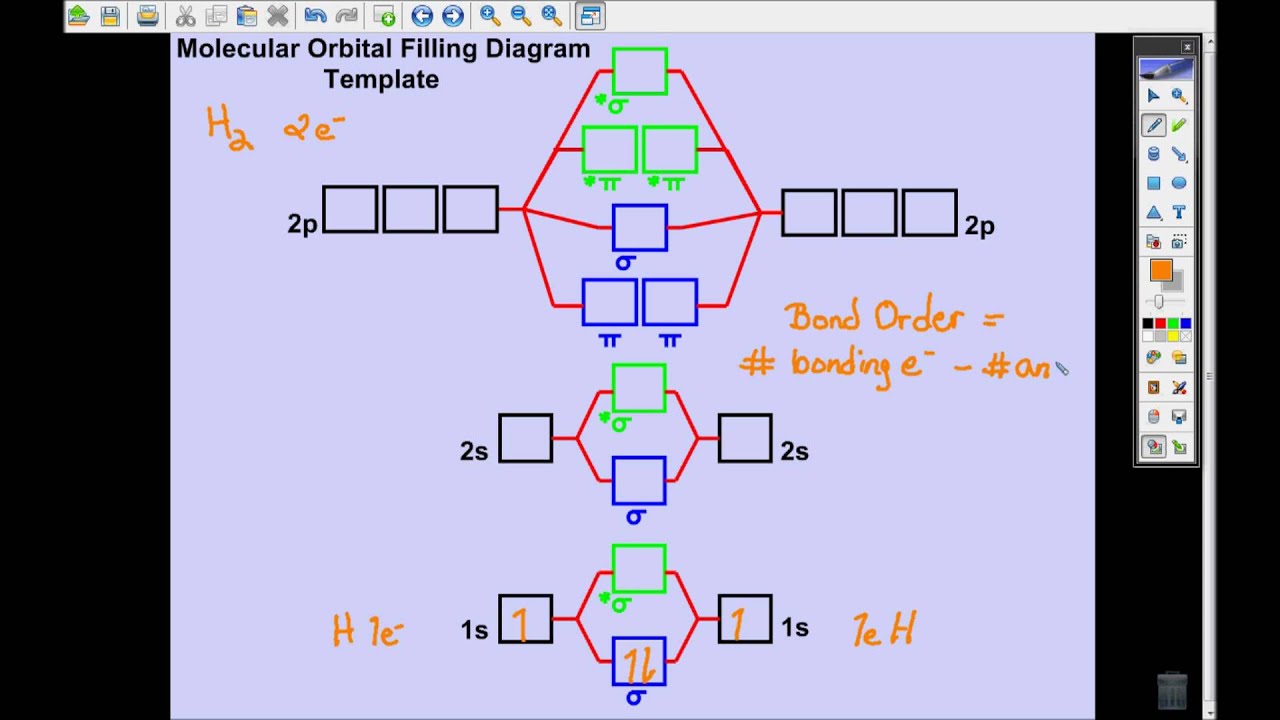
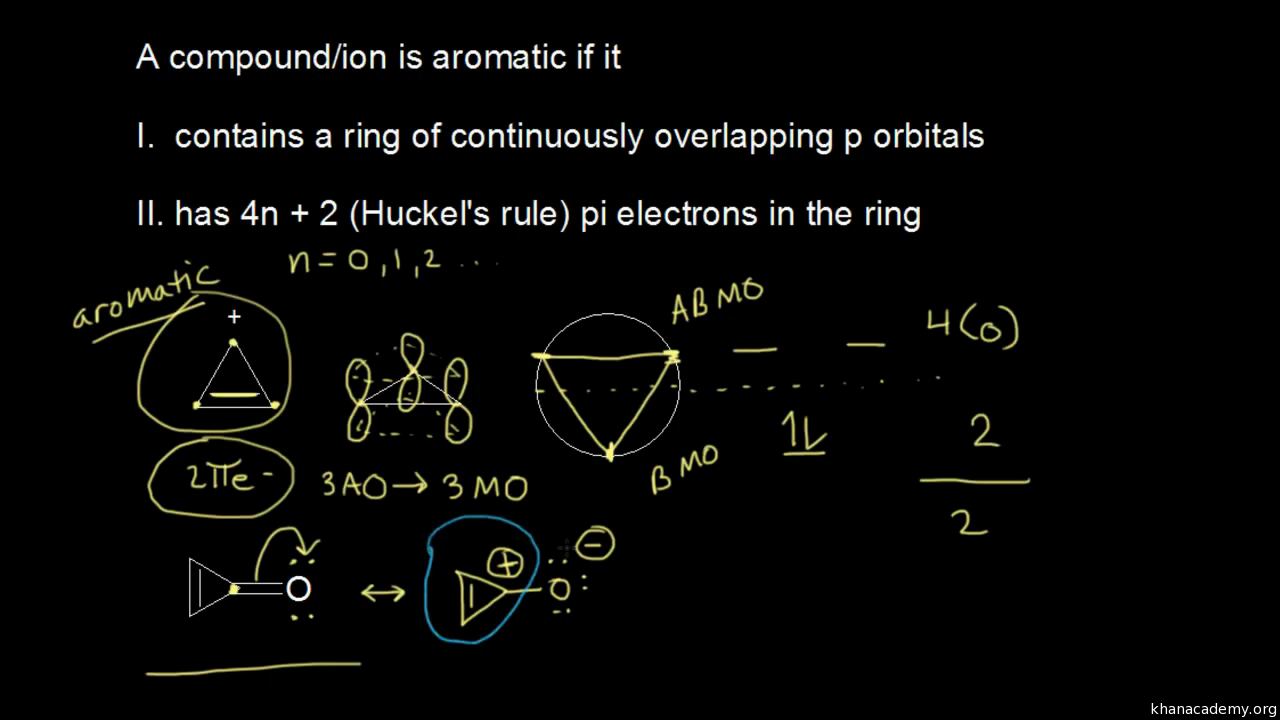
Khan Academy Molecular Orbital Theory
Khan Academy Molecular Orbital Theory - Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. If you're seeing this message, it means we're having trouble loading external resources on our website. This is a great question that goes into the details of molecular orbital theory and chemical bonding. In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. When a nitrogen atom forms an ammonia. If you're behind a web filter, please.
When a nitrogen atom forms an ammonia. If you're seeing this message, it means we're having trouble loading external resources on our website. Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. This is a great question that goes into the details of molecular orbital theory and chemical bonding. If you're behind a web filter, please. In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence.
If you're seeing this message, it means we're having trouble loading external resources on our website. When a nitrogen atom forms an ammonia. If you're behind a web filter, please. This is a great question that goes into the details of molecular orbital theory and chemical bonding. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy.
Molecular Orbital Theory (MOT)→ molecular orbital theory explains abou..
In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. If you're seeing this message, it means we're having trouble loading external resources on our.
how to draw molecular orbital diagram khan academy Lourie Thatcher
When a nitrogen atom forms an ammonia. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. If you're seeing this message, it means we're.
Understanding Molecular Orbital Diagrams with Khan Academy
If you're behind a web filter, please. In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. If you're seeing this message, it means we're having trouble loading external resources on our website. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of.
The Complete Guide to Understanding Molecular Orbital Diagrams on Khan
Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. When a nitrogen atom forms an ammonia. This is a great question that goes into the details of molecular orbital theory and chemical.
3 Pi Bonds And Sp2 Hybridized Orbitals Video Khan Academy Khan Academy
In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. If you're behind a web filter, please. When a nitrogen atom forms an ammonia. Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. In mo theory explaining bonding, anti bonding, and non.
Understanding Khan Academy's Orbital Diagrams A Comprehensive Guide
Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. If you're seeing this message, it means we're having trouble loading external resources on our website. When a nitrogen atom forms an ammonia. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence..
Molecular Orbital Diagram Khan Academy Hanenhuusholli
Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please. This is a great question that goes into the details of molecular orbital theory and chemical bonding. In molecular orbital theory, a covalent.
The Ultimate Guide to Understanding Orbital Diagrams A Khan Academy
This is a great question that goes into the details of molecular orbital theory and chemical bonding. If you're behind a web filter, please. If you're seeing this message, it means we're having trouble loading external resources on our website. Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. In molecular orbital theory, a covalent.
Molecular Orbital Theory, Part 2 YouTube
This is a great question that goes into the details of molecular orbital theory and chemical bonding. In molecular orbital theory, a covalent bond is formed whenever two atoms overlap all of their orbitals, regardless of whether they are valence. If you're behind a web filter, please. In mo theory explaining bonding, anti bonding, and non bonding orbitals in general.
Molecular Orbital Diagram Khan Academy Hanenhuusholli
In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. This is a great question that goes into the details of molecular orbital theory and chemical bonding. Explore advanced applications of molecular orbital theory in this comprehensive guide on khan academy. When a nitrogen atom forms an ammonia..
In Molecular Orbital Theory, A Covalent Bond Is Formed Whenever Two Atoms Overlap All Of Their Orbitals, Regardless Of Whether They Are Valence.
In mo theory explaining bonding, anti bonding, and non bonding orbitals in general and how to fill the electrons in the orbitals. If you're seeing this message, it means we're having trouble loading external resources on our website. This is a great question that goes into the details of molecular orbital theory and chemical bonding. When a nitrogen atom forms an ammonia.
Explore Advanced Applications Of Molecular Orbital Theory In This Comprehensive Guide On Khan Academy.
If you're behind a web filter, please.