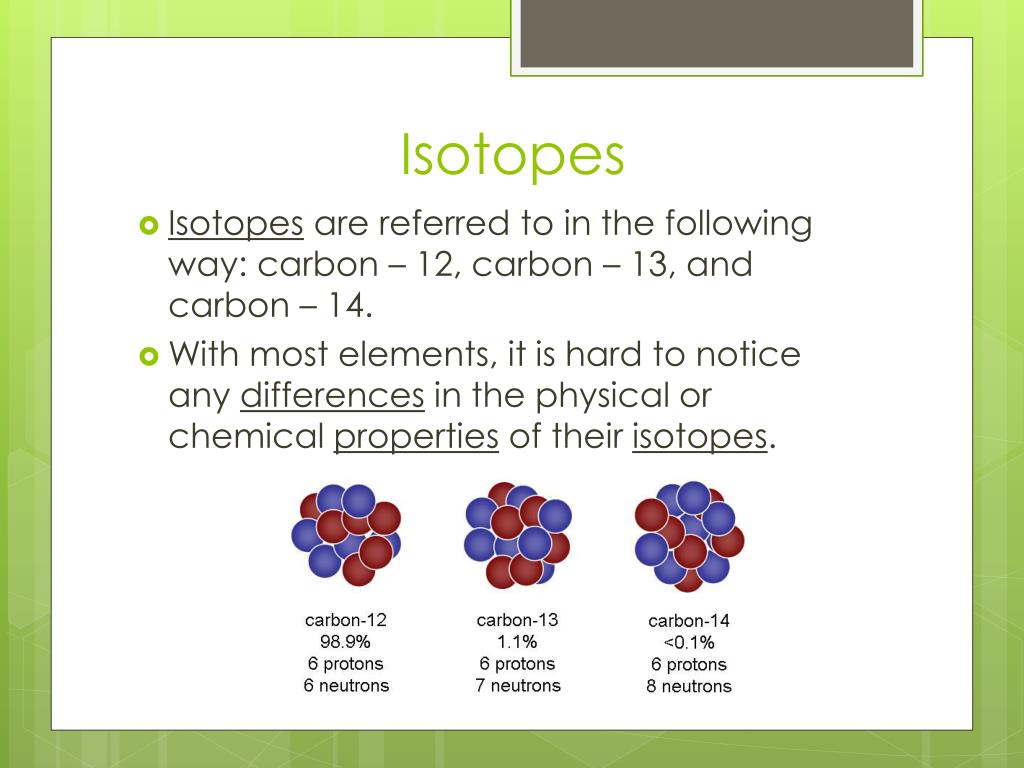
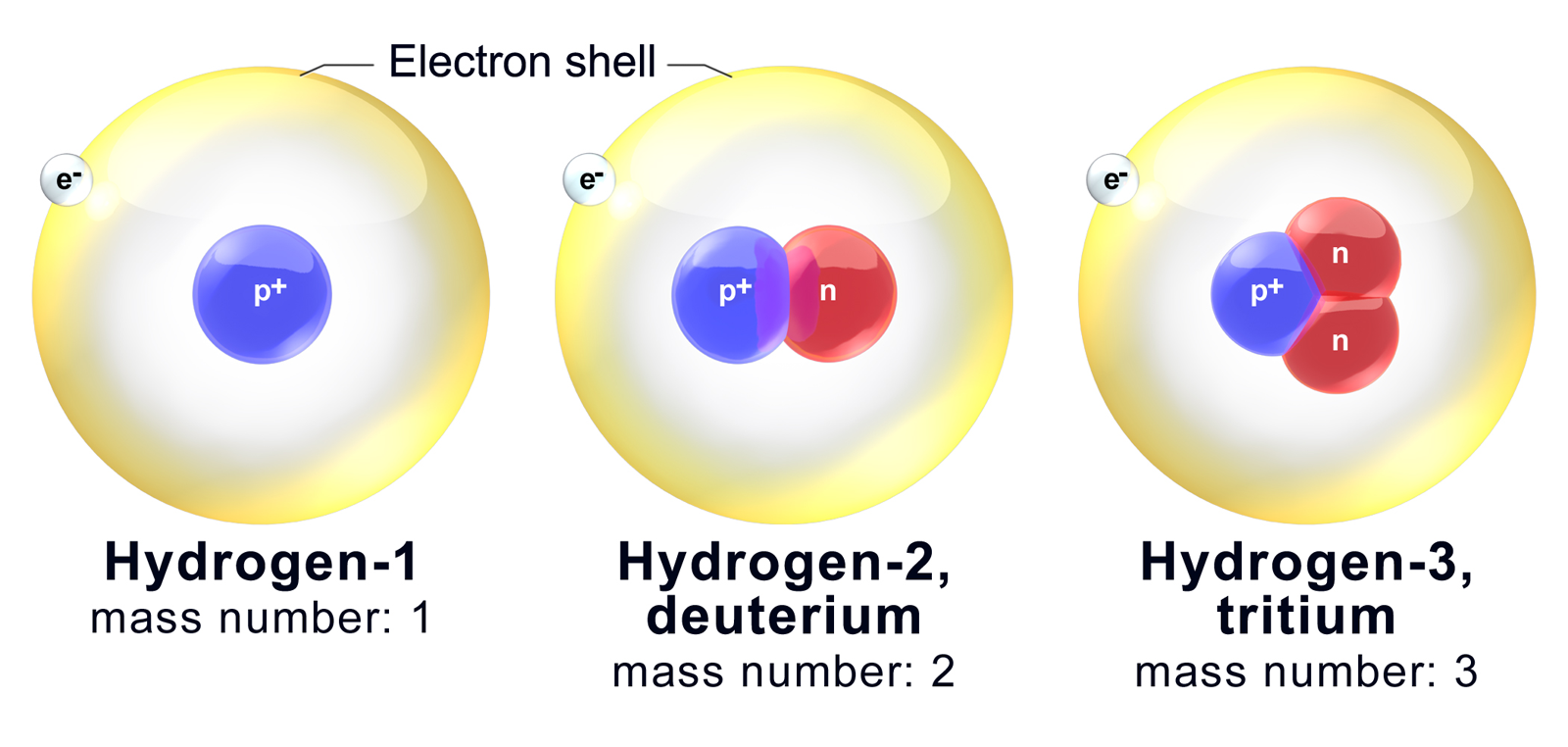
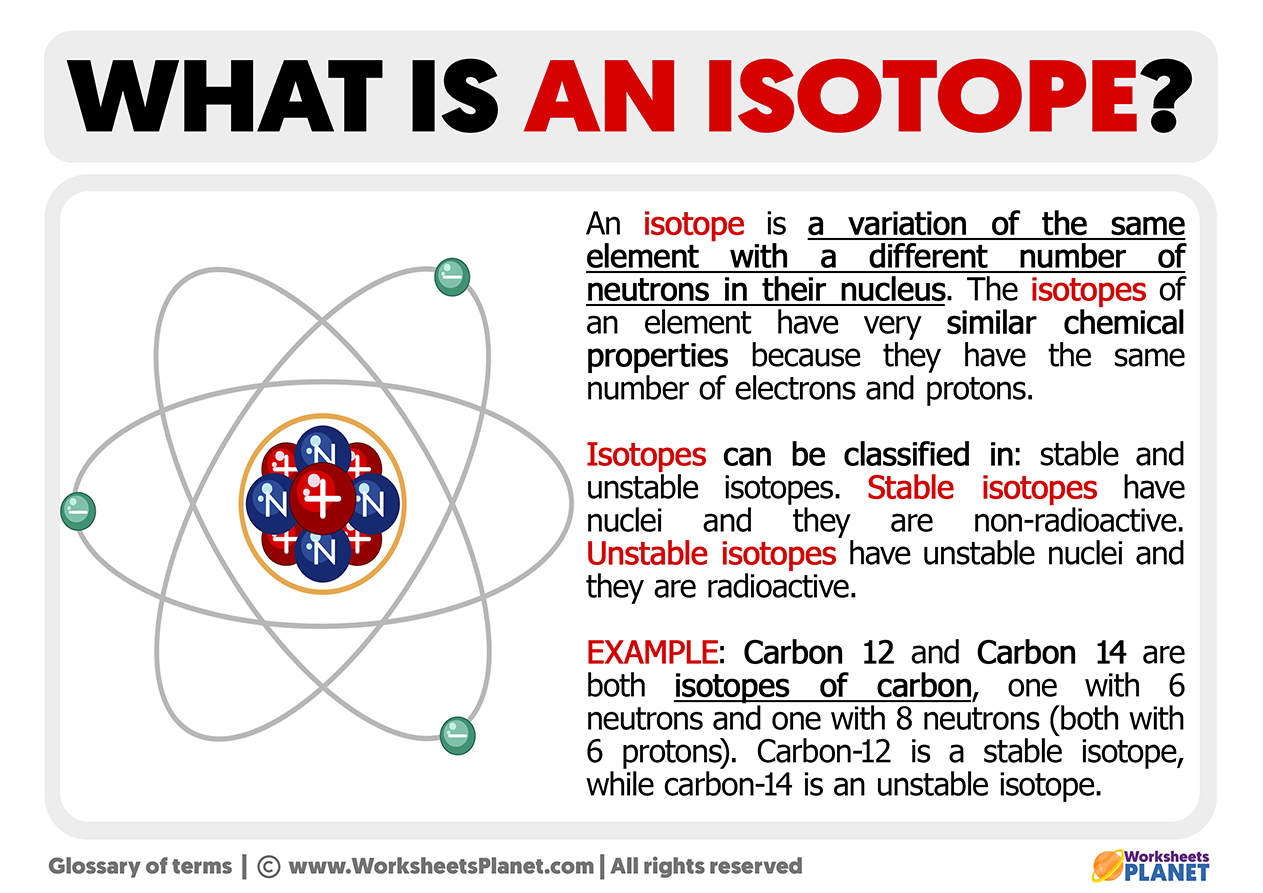
Isotopes Are Different Forms Of The Same Element That
Isotopes Are Different Forms Of The Same Element That - Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that contain different numbers of neutrons.
This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in.
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
Definition Of Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount.
Definition and Examples of Isotopes in Chemistry
This difference in neutron amount. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. Isotopes are atoms of the same element that contain different numbers of neutrons.
Isotopes and Ions Seventh Grade Science. ppt download
Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
PPT Chapter 4 Atomic Structure PowerPoint Presentation, free download
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses.
DOE Explains...Isotopes Department of Energy
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount. Isotopes are atoms of the same element that contain different numbers of neutrons.
"Isotopes and their uses"
Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in.
2 Naturally Occuring Isotopes For Lithium Chemistry International
Isotopes are atoms of the same element that contain different numbers of neutrons. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. This difference in neutron amount.
PPT Understanding Isotopes and Their Importance in Chemistry
Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount.
What is an Isotope Definition of Isotope
Isotopes are atoms of the same element that contain different numbers of neutrons. 289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount.
Compound Interest ChemistryAdvent IYPT2019 Day 20 A periodic table
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. Isotopes are atoms of the same element that contain different numbers of neutrons. This difference in neutron amount.
Isotopes Are Atoms Of The Same Element That Contain Different Numbers Of Neutrons.
289 rows an isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in. Isotopes are atoms of the same element that have different numbers of neutrons and, therefore, different masses. This difference in neutron amount.

/Isotope-58dd6b415f9b5846830254ae.jpg)
.jpg)