Heating And Cooling Curves Worksheet
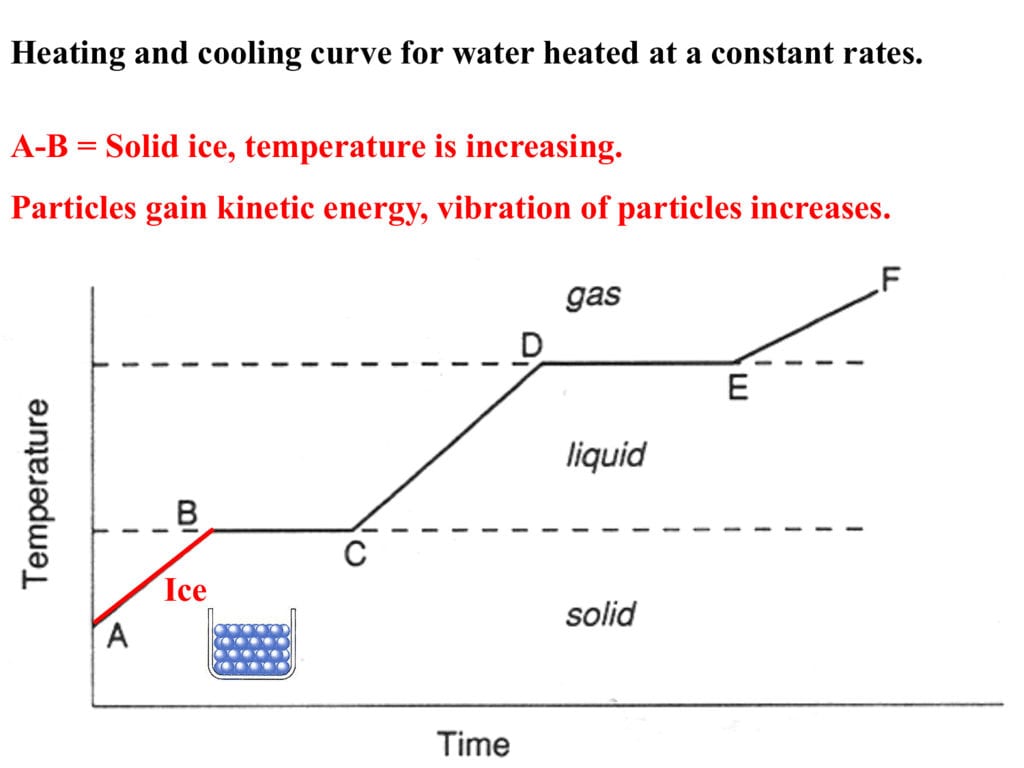
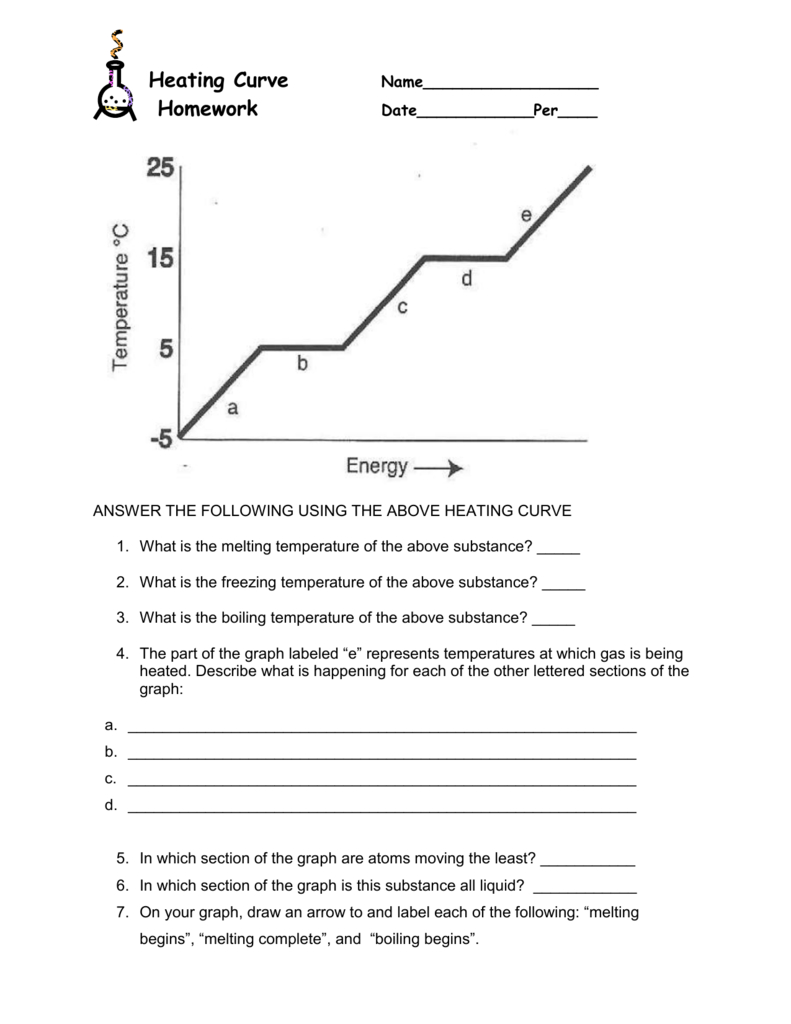
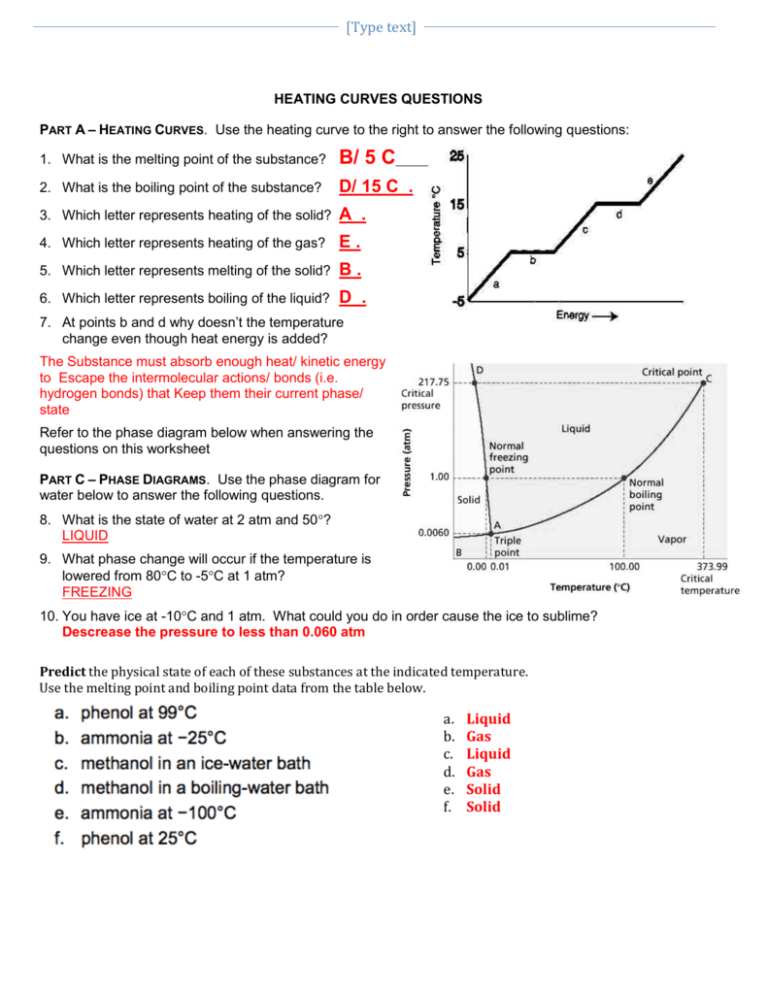
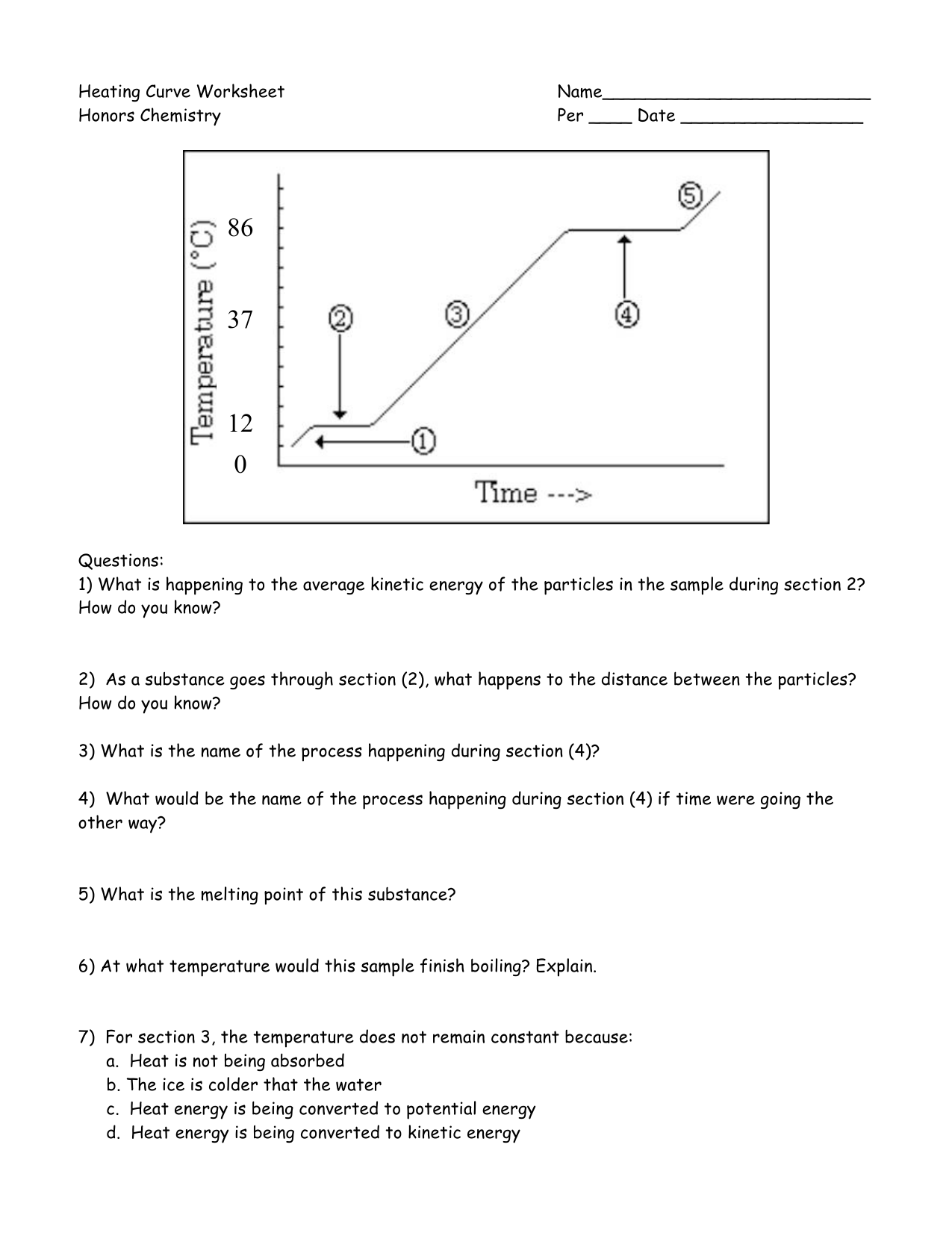
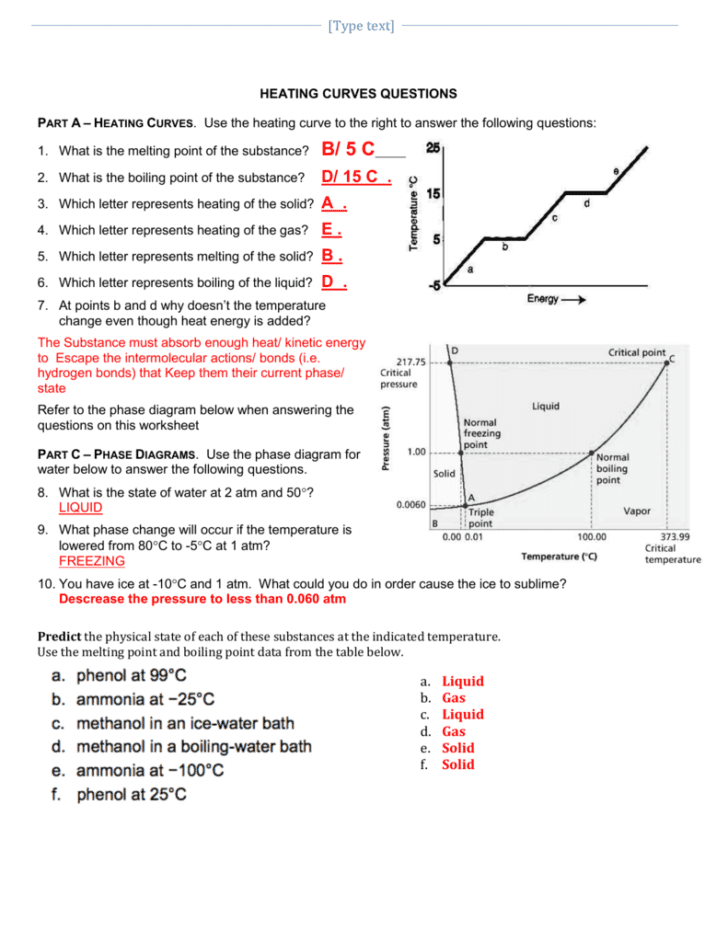
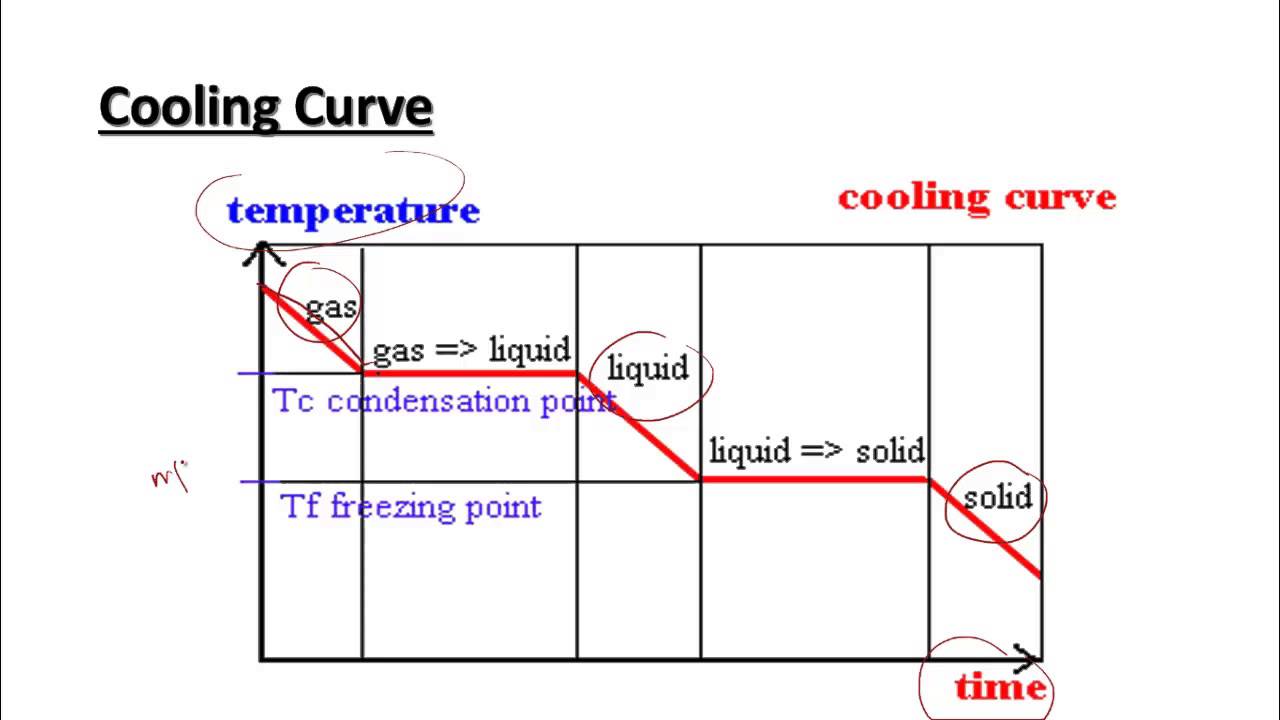
Heating And Cooling Curves Worksheet - It represents the heating of substance x at a constant rate of. I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of ice. Use dimensional analysis or the specific heat equation to complete the following problems. Heating and cooling curves target: _____ figure 1 figure 1shows the temperature of 1.00. The heating curve shown above is a plot of temperature vs time.
I can use heating and cooling curves to help calculate the energy changes during phase changes Heating and cooling curves target: _____ figure 1 figure 1shows the temperature of 1.00. Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice. The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of.
Heating and cooling curves target: _____ figure 1 figure 1shows the temperature of 1.00. Use dimensional analysis or the specific heat equation to complete the following problems. I can use heating and cooling curves to help calculate the energy changes during phase changes It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time. How much heat is required to melt 25.0 g of ice.
Heating Cooling Curve Worksheet Answers —
The heating curve shown above is a plot of temperature vs time. I can use heating and cooling curves to help calculate the energy changes during phase changes How much heat is required to melt 25.0 g of ice. It represents the heating of substance x at a constant rate of. Heating and cooling curves target:
The Ultimate Guide to Understanding Worksheet 1 Heating and Cooling
Use dimensional analysis or the specific heat equation to complete the following problems. _____ figure 1 figure 1shows the temperature of 1.00. The heating curve shown above is a plot of temperature vs time. Heating and cooling curves target: I can use heating and cooling curves to help calculate the energy changes during phase changes
Heating And Cooling Curves Worksheet —
The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of. How much heat is required to melt 25.0 g of ice. _____ figure 1 figure 1shows the temperature of 1.00. Heating and cooling curves target:
Heating And Cooling Curves Worksheets
The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of. Use dimensional analysis or the specific heat equation to complete the following problems. I can use heating and cooling curves to help calculate the energy changes during phase changes Heating and cooling curves target:
Understanding Heating And Cooling Curves Worksheet
How much heat is required to melt 25.0 g of ice. _____ figure 1 figure 1shows the temperature of 1.00. It represents the heating of substance x at a constant rate of. Heating and cooling curves target: Use dimensional analysis or the specific heat equation to complete the following problems.
Heating Curves Worksheet Answers Printable Word Searches
How much heat is required to melt 25.0 g of ice. Heating and cooling curves target: Use dimensional analysis or the specific heat equation to complete the following problems. _____ figure 1 figure 1shows the temperature of 1.00. The heating curve shown above is a plot of temperature vs time.
Heating And Cooling Curves Worksheet Educational worksheets
It represents the heating of substance x at a constant rate of. Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g of ice. _____ figure 1 figure 1shows the temperature of 1.00. I can use heating and cooling curves to help calculate the energy changes during phase.
Heating Curve Worksheet Worksheet
The heating curve shown above is a plot of temperature vs time. I can use heating and cooling curves to help calculate the energy changes during phase changes Use dimensional analysis or the specific heat equation to complete the following problems. _____ figure 1 figure 1shows the temperature of 1.00. How much heat is required to melt 25.0 g of.
Heating Cooling Curve Worksheet
How much heat is required to melt 25.0 g of ice. The heating curve shown above is a plot of temperature vs time. I can use heating and cooling curves to help calculate the energy changes during phase changes Use dimensional analysis or the specific heat equation to complete the following problems. It represents the heating of substance x at.
39 heating and cooling curves worksheet Worksheet Resource
Heating and cooling curves target: How much heat is required to melt 25.0 g of ice. Use dimensional analysis or the specific heat equation to complete the following problems. The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of.
Heating And Cooling Curves Target:
Use dimensional analysis or the specific heat equation to complete the following problems. _____ figure 1 figure 1shows the temperature of 1.00. I can use heating and cooling curves to help calculate the energy changes during phase changes The heating curve shown above is a plot of temperature vs time.
How Much Heat Is Required To Melt 25.0 G Of Ice.
It represents the heating of substance x at a constant rate of.