Fda Prior Notice Form Fedex
Fda Prior Notice Form Fedex - Food and drug administration (fda), stating that the. One to support the registration of facilities that manufacture, process, pack, or. The act requires that fda develop two systems: Submitter, importer and carrier information. On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to. In order to send food products to the u.s., the exporter must file a prior notice with the u.s. Importing gift packs and prior notice; Prior notice policy on sending gifts to your friends and family; For a new prior notice submission, select create. Creating a prior notice is a 2 steps process.
Prior notice policy on sending gifts to your friends and family; Importing gift packs and prior notice; One to support the registration of facilities that manufacture, process, pack, or. For a new prior notice submission, select create. In order to send food products to the u.s., the exporter must file a prior notice with the u.s. Submitter, importer and carrier information. Creating a prior notice is a 2 steps process. Food and drug administration (fda), stating that the. The act requires that fda develop two systems: On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to.
For a new prior notice submission, select create. On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to. One to support the registration of facilities that manufacture, process, pack, or. Importing gift packs and prior notice; Submitter, importer and carrier information. The act requires that fda develop two systems: In order to send food products to the u.s., the exporter must file a prior notice with the u.s. Food and drug administration (fda), stating that the. Creating a prior notice is a 2 steps process. Prior notice policy on sending gifts to your friends and family;
Prior Notice System Interface (PNSI) StepbyStep Instructions
Food and drug administration (fda), stating that the. Prior notice policy on sending gifts to your friends and family; Importing gift packs and prior notice; In order to send food products to the u.s., the exporter must file a prior notice with the u.s. On may 5, 2011 the fda published an interim final rule requiring that a person submitting.
FDA Prior Notice Filings Ethical Fashion Guatemala
For a new prior notice submission, select create. One to support the registration of facilities that manufacture, process, pack, or. The act requires that fda develop two systems: In order to send food products to the u.s., the exporter must file a prior notice with the u.s. Prior notice policy on sending gifts to your friends and family;
Fedex Registration Form Fill Online, Printable, Fillable, Blank
Food and drug administration (fda), stating that the. Importing gift packs and prior notice; Creating a prior notice is a 2 steps process. Submitter, importer and carrier information. The act requires that fda develop two systems:
Prior Notice Basics
One to support the registration of facilities that manufacture, process, pack, or. In order to send food products to the u.s., the exporter must file a prior notice with the u.s. Importing gift packs and prior notice; Submitter, importer and carrier information. The act requires that fda develop two systems:
US FDA Prior Notice Form_First Choice Consulting Services PDF
Submitter, importer and carrier information. One to support the registration of facilities that manufacture, process, pack, or. Importing gift packs and prior notice; On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to. Creating a prior notice is a 2 steps process.
Sample FDA Prior Notices FDA Prior Notice Submission Service for
On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to. One to support the registration of facilities that manufacture, process, pack, or. Importing gift packs and prior notice; The act requires that fda develop two systems: Prior notice policy on sending gifts to your.
Sample FDA Prior Notices FDA Prior Notice Submission Service for
For a new prior notice submission, select create. Creating a prior notice is a 2 steps process. Prior notice policy on sending gifts to your friends and family; Food and drug administration (fda), stating that the. In order to send food products to the u.s., the exporter must file a prior notice with the u.s.
Automate Your FDA Prior Notices With PriorNotify
Creating a prior notice is a 2 steps process. On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to. Prior notice policy on sending gifts to your friends and family; Importing gift packs and prior notice; The act requires that fda develop two systems:
FDA Prior Notices There's an App for That! Insights
Creating a prior notice is a 2 steps process. For a new prior notice submission, select create. Importing gift packs and prior notice; Submitter, importer and carrier information. The act requires that fda develop two systems:
US FDA Prior Notice Form_First Choice Consulting Services
Food and drug administration (fda), stating that the. Prior notice policy on sending gifts to your friends and family; Submitter, importer and carrier information. For a new prior notice submission, select create. Importing gift packs and prior notice;
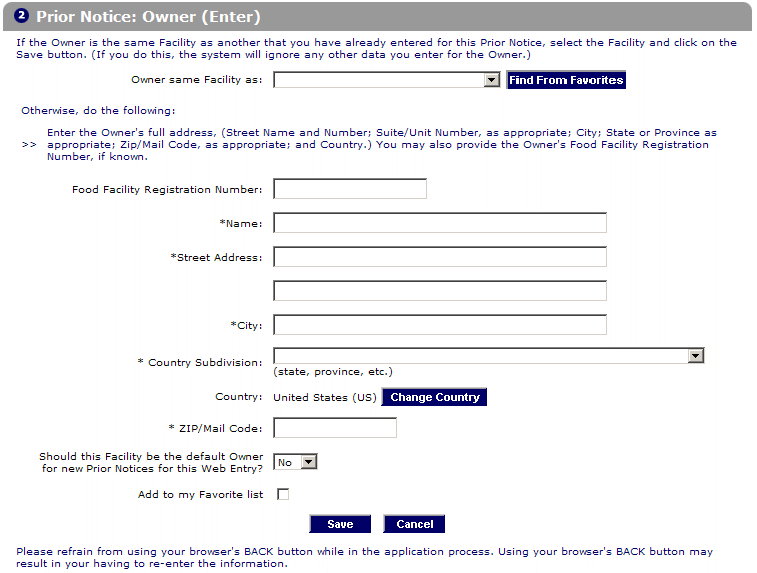
For A New Prior Notice Submission, Select Create.
Importing gift packs and prior notice; Food and drug administration (fda), stating that the. In order to send food products to the u.s., the exporter must file a prior notice with the u.s. Prior notice policy on sending gifts to your friends and family;
Creating A Prior Notice Is A 2 Steps Process.
The act requires that fda develop two systems: On may 5, 2011 the fda published an interim final rule requiring that a person submitting prior notice of imported food, including food for animals, to. One to support the registration of facilities that manufacture, process, pack, or. Submitter, importer and carrier information.