Fda Diversity Plan Template
Fda Diversity Plan Template - The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. In the april 2022 draft guidance, the fda outlined a policy whereby sponsors of certain. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. The diversity plan guidance in a nutshell: Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from. Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device.
The diversity plan guidance in a nutshell: Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from. In the april 2022 draft guidance, the fda outlined a policy whereby sponsors of certain. Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to.
In the april 2022 draft guidance, the fda outlined a policy whereby sponsors of certain. The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to. Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. The diversity plan guidance in a nutshell: Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from.
Fda Diversity Plan Template
Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to. The diversity plan guidance in.
FDA Perspectives on Diversity in Clinical Trials
The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to. Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from. In the april.
Diversity Plan Template Printable Word Searches
Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. The united states food and drug.
Fda Diversity Plan Template
Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. In the april 2022 draft guidance, the fda outlined a policy whereby sponsors of certain. The diversity plan guidance in a nutshell: Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. This.
Fda Diversity Plan Template
This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to. Last month, congress took a.
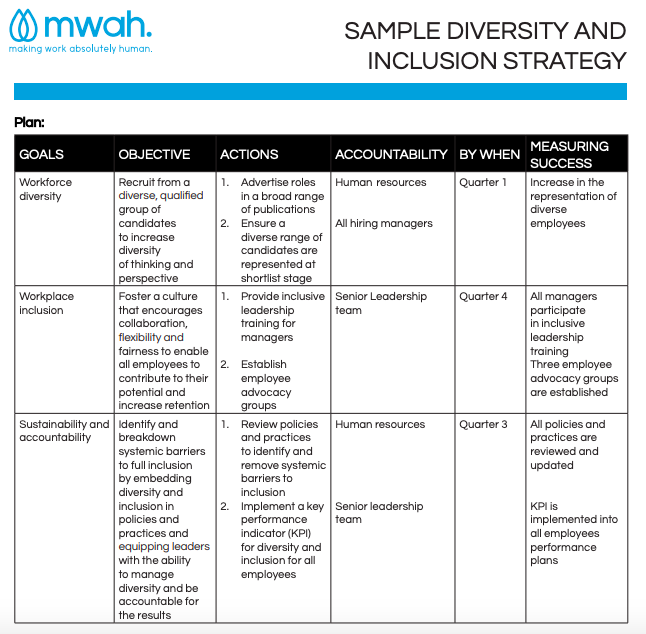
Diversity And Inclusion Plan Template
The diversity plan guidance in a nutshell: In the april 2022 draft guidance, the fda outlined a policy whereby sponsors of certain. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to..
Fda Diversity Plan Template
This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. The diversity plan guidance.
Fda Diversity Plan Template
Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. In the april 2022 draft guidance, the fda outlined a policy whereby sponsors of certain. This draft guidance describes the form, content, and.
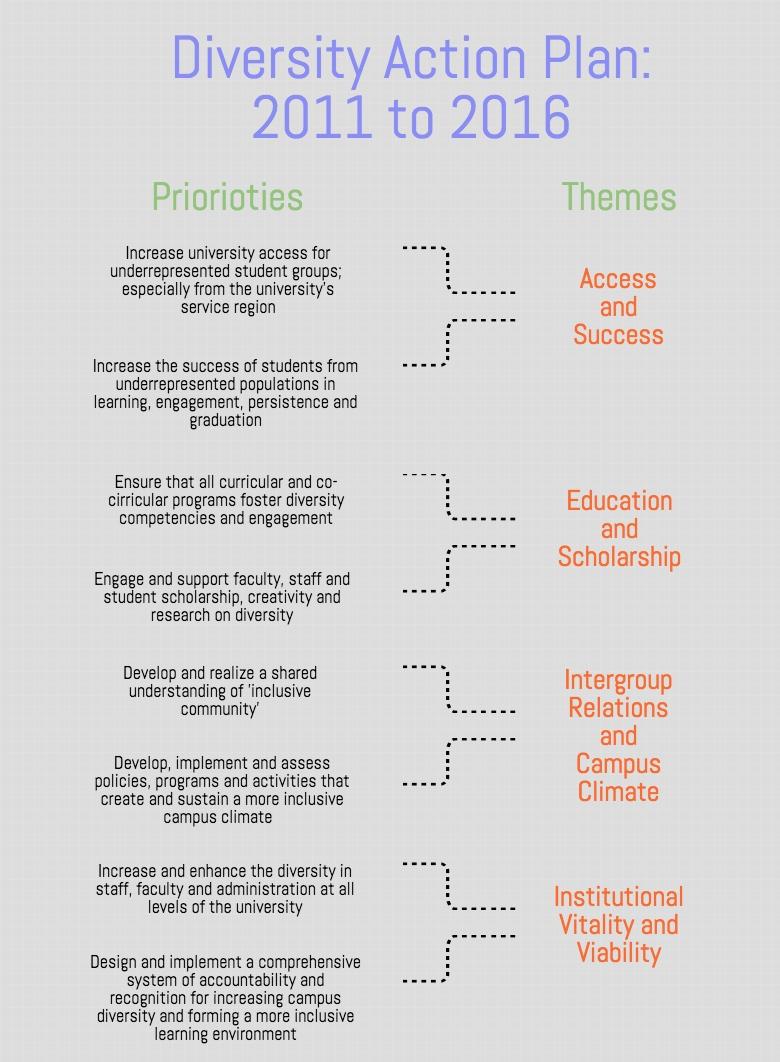
Diversity And Inclusion Action Plan Template
Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. This draft guidance describes the form, content, and manner of diversity action plans, the applicable medical products, and clinical. In the april 2022.
FDA Draft Guidance on Enhancing the Diversity of Clinical Trial
The diversity plan guidance in a nutshell: The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most.
This Draft Guidance Describes The Form, Content, And Manner Of Diversity Action Plans, The Applicable Medical Products, And Clinical.
The diversity plan guidance in a nutshell: Food and drug administration issued a draft guidance, “ diversity action plans to improve enrollment of participants from. Given the importance of increasing enrollment from historically underrepresented racial and ethnic populations, fda is publishing this. The united states food and drug administration (fda) issued draft guidance on april 13, 2022, entitled, “diversity plans to.
In The April 2022 Draft Guidance, The Fda Outlined A Policy Whereby Sponsors Of Certain.
Last month, congress took a big step towards improving clinical trial diversity by requiring sponsors of most drug and device.