Clinical Trial Application Form
Clinical Trial Application Form - This page provides links to commonly used clinical trial forms relevant to clinical trials. Provide an introduction and background information. Describe past experimental and/or clinical findings leading to. Learn how to be sure you are using the right. Does your human subjects study meet the nih definition of a clinical trial? Interventional studies (also called clinical trials) and observational studies. For other fda forms, visit the fda forms page. There are two types of clinical studies: The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into.
The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. There are two types of clinical studies: Describe past experimental and/or clinical findings leading to. Learn how to be sure you are using the right. For other fda forms, visit the fda forms page. Provide an introduction and background information. Interventional studies (also called clinical trials) and observational studies. Does your human subjects study meet the nih definition of a clinical trial? This page provides links to commonly used clinical trial forms relevant to clinical trials.
Describe past experimental and/or clinical findings leading to. Provide an introduction and background information. Learn how to be sure you are using the right. There are two types of clinical studies: Does your human subjects study meet the nih definition of a clinical trial? Interventional studies (also called clinical trials) and observational studies. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. This page provides links to commonly used clinical trial forms relevant to clinical trials. For other fda forms, visit the fda forms page.
Clinical Trial Approval Process In Canada Credevo Articles
Interventional studies (also called clinical trials) and observational studies. Provide an introduction and background information. Describe past experimental and/or clinical findings leading to. For other fda forms, visit the fda forms page. There are two types of clinical studies:
Fillable Online Application Form for a New Clinical Trial.doc Fax Email
Provide an introduction and background information. There are two types of clinical studies: For other fda forms, visit the fda forms page. Interventional studies (also called clinical trials) and observational studies. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into.
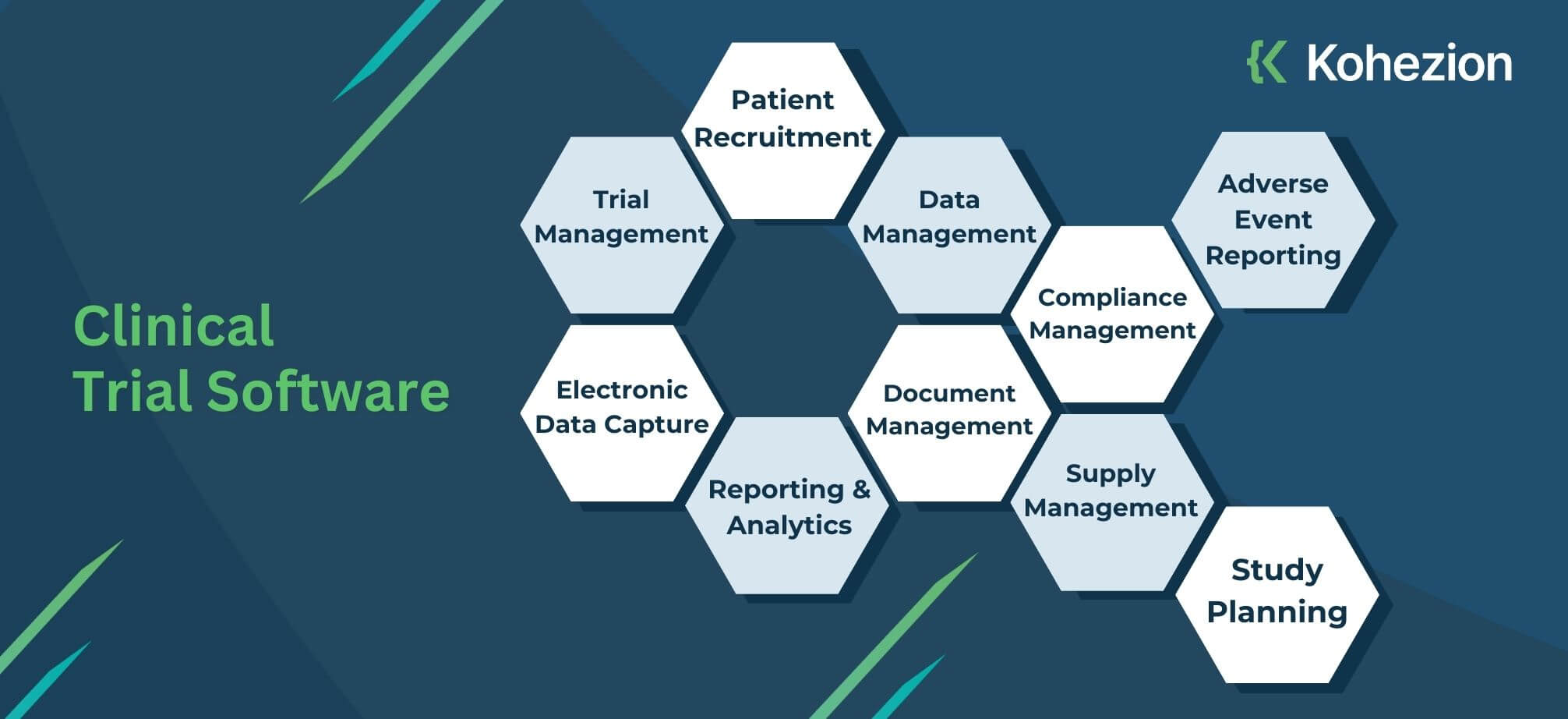
Custom Clinical Trial Software
There are two types of clinical studies: The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. This page provides links to commonly used clinical trial forms relevant to clinical trials. Does your human subjects study meet the nih definition of a clinical trial? For other fda forms, visit the fda forms.
UK MHRA Guidance Clinical Trials for Medicines Apply for
For other fda forms, visit the fda forms page. Does your human subjects study meet the nih definition of a clinical trial? Learn how to be sure you are using the right. There are two types of clinical studies: Describe past experimental and/or clinical findings leading to.
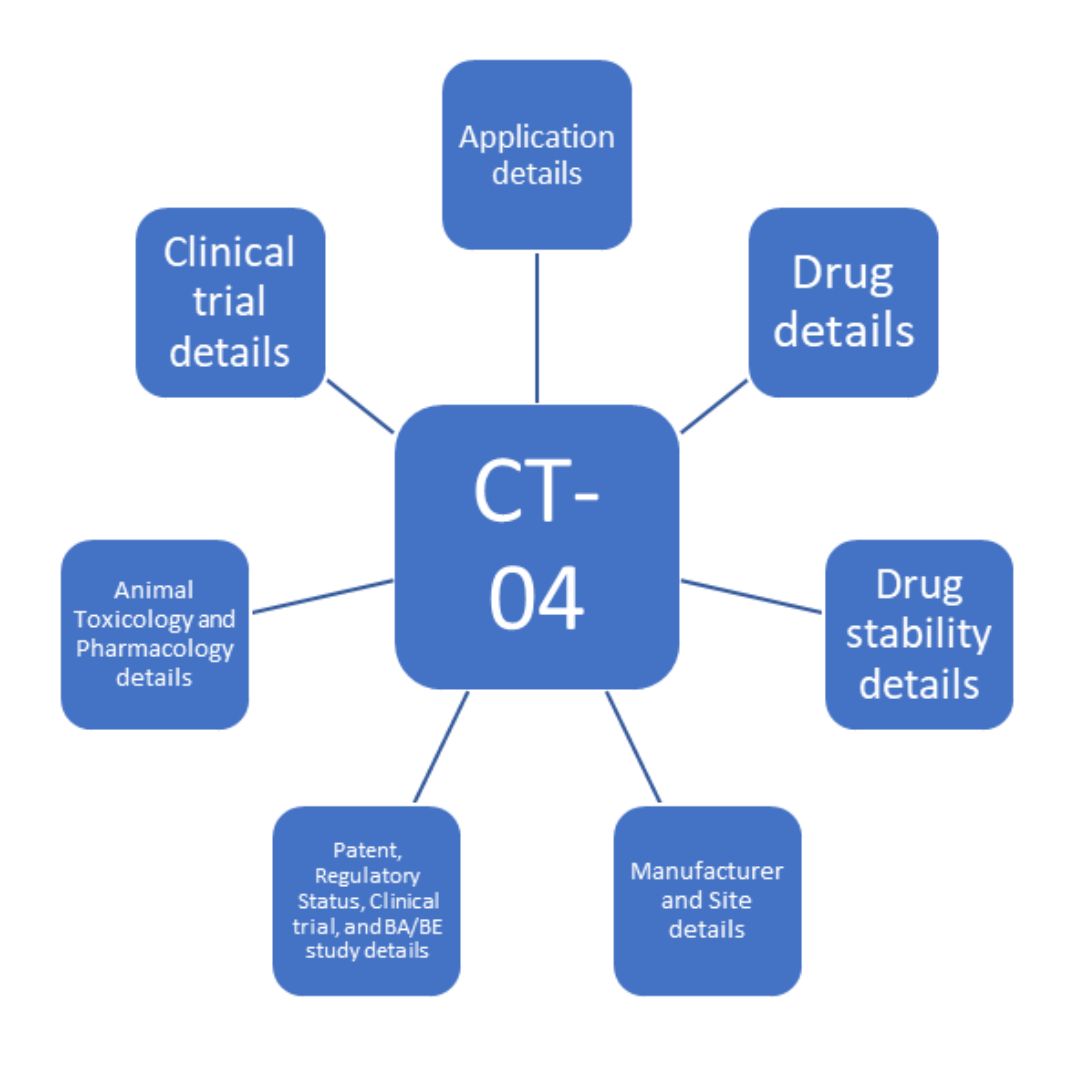
C IRB Clinical Trial Application Form
Provide an introduction and background information. Interventional studies (also called clinical trials) and observational studies. This page provides links to commonly used clinical trial forms relevant to clinical trials. Does your human subjects study meet the nih definition of a clinical trial? Describe past experimental and/or clinical findings leading to.
Clinical Trial Registration and Application Form Clinical Research
Learn how to be sure you are using the right. Provide an introduction and background information. Does your human subjects study meet the nih definition of a clinical trial? The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Interventional studies (also called clinical trials) and observational studies.
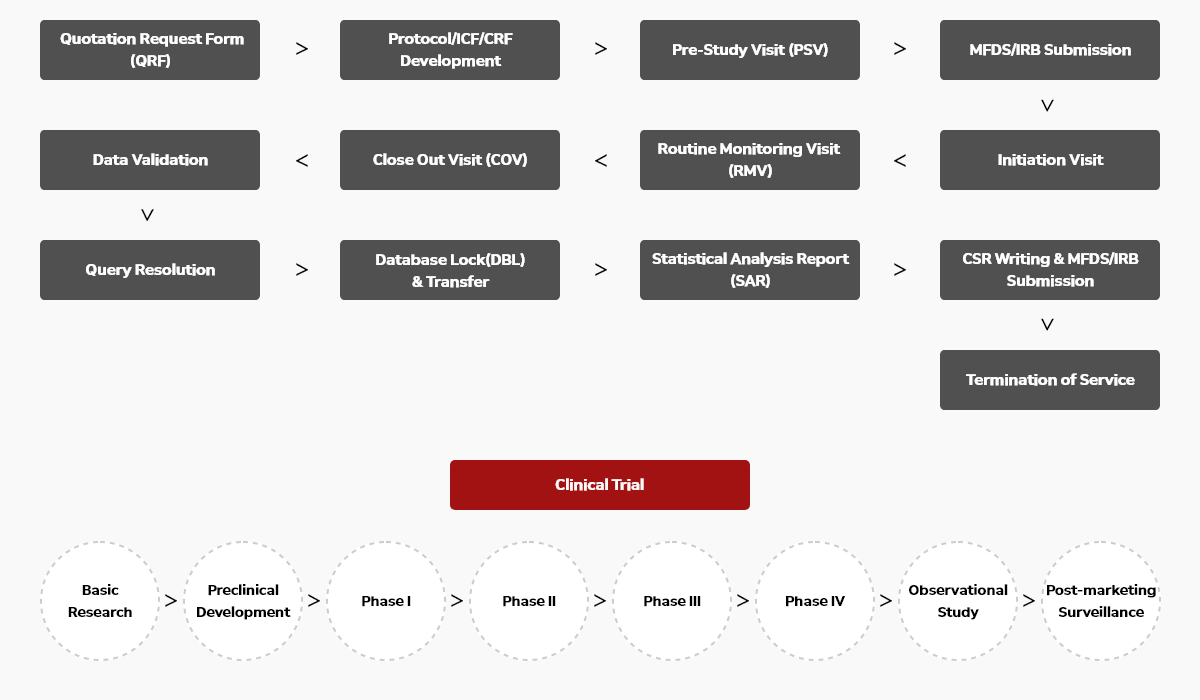
Clinical Trial Process intoinworld
Describe past experimental and/or clinical findings leading to. Interventional studies (also called clinical trials) and observational studies. Provide an introduction and background information. There are two types of clinical studies: This page provides links to commonly used clinical trial forms relevant to clinical trials.
Clinical Trial Application and Import Requirements in India with
The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Does your human subjects study meet the nih definition of a clinical trial? For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials. There are two types of clinical.
Fillable Online Clinical trial Application Form Europa Fax Email
For other fda forms, visit the fda forms page. Interventional studies (also called clinical trials) and observational studies. Learn how to be sure you are using the right. Does your human subjects study meet the nih definition of a clinical trial? There are two types of clinical studies:
Clinical Trial Application Form Fill Online, Printable, Fillable
This page provides links to commonly used clinical trial forms relevant to clinical trials. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into. Interventional studies (also called clinical trials) and observational studies. Describe past experimental and/or clinical findings leading to. Provide an introduction and background information.
For Other Fda Forms, Visit The Fda Forms Page.
Learn how to be sure you are using the right. This page provides links to commonly used clinical trial forms relevant to clinical trials. Does your human subjects study meet the nih definition of a clinical trial? Interventional studies (also called clinical trials) and observational studies.
There Are Two Types Of Clinical Studies:
Describe past experimental and/or clinical findings leading to. Provide an introduction and background information. The phs human subjects and clinical trial form consolidates human subjects, inclusion enrollment, and clinical trial information into.