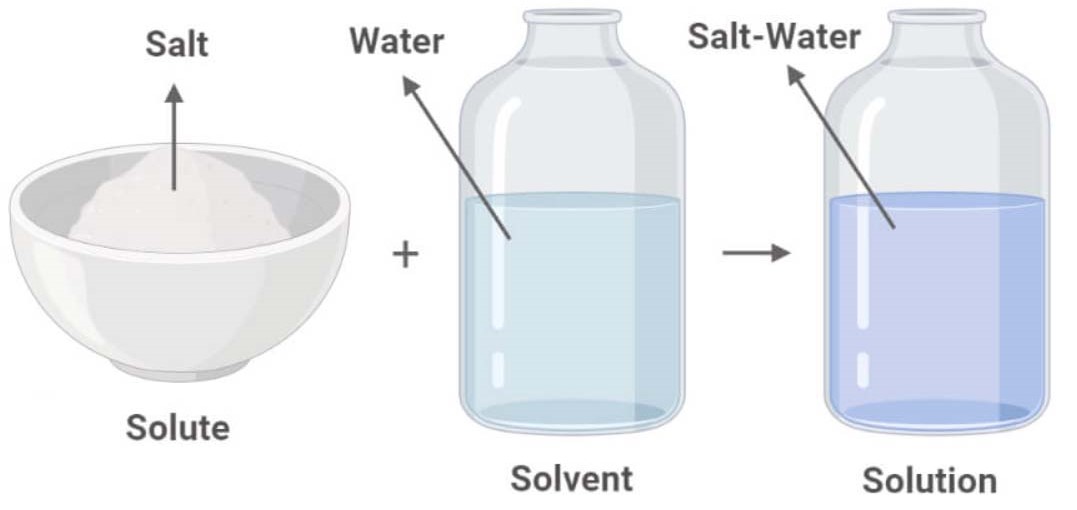
A Salt Will Dissolve In Water To Form
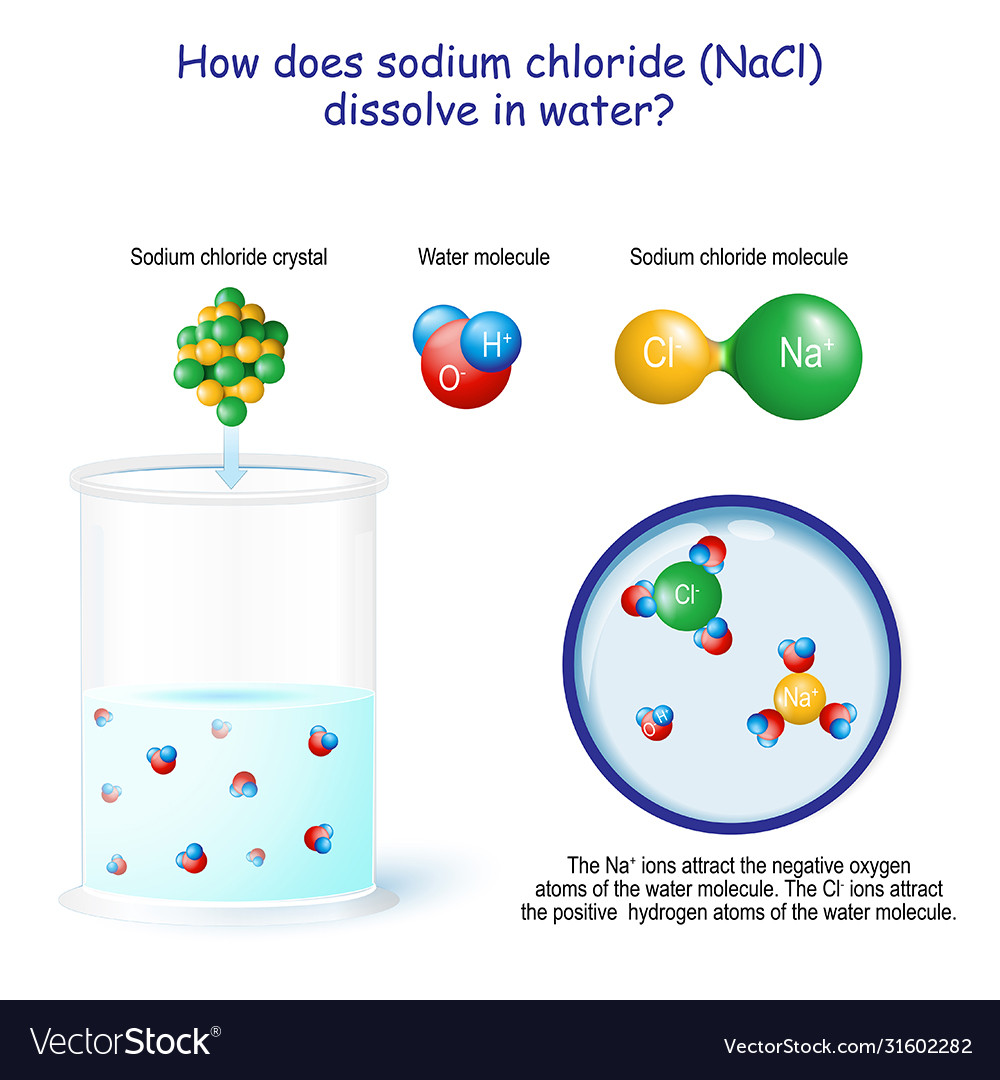
A Salt Will Dissolve In Water To Form - This process is called hydration,. In chemistry, it results in a solution, as the ionic bond of nacl. Salt dissolves in water due to the polar nature of both substances. Salt dissolved in water is a rough description of earth's oceans. Water is a polar molecule, which means it has a slightly. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. A salt will dissolve in water to form. Four of the five answers listed below are characteristics of water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Substances that are ____ give up hydrogen ions when they dissolve in water.
In chemistry, it results in a solution, as the ionic bond of nacl. This process is called hydration,. Four of the five answers listed below are characteristics of water. Salt dissolves in water due to the polar nature of both substances. Substances that are ____ give up hydrogen ions when they dissolve in water. When dissolved in water, a. Salt dissolved in water is a rough description of earth's oceans. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. A salt will dissolve in water to form.
When dissolved in water, a. Four of the five answers listed below are characteristics of water. This process is called hydration,. A salt will dissolve in water to form. Salt dissolved in water is a rough description of earth's oceans. Salt dissolves in water due to the polar nature of both substances. Substances that are ____ give up hydrogen ions when they dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Water is a polar molecule, which means it has a slightly. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are.
Ions In Aqueous Solution Infographic Diagram Showing, 44 OFF
Four of the five answers listed below are characteristics of water. When dissolved in water, a. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Water is a polar molecule, which means it has a slightly. Salt dissolves in water due to the polar nature.
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt
Salt dissolves in water due to the polar nature of both substances. Water is a polar molecule, which means it has a slightly. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Four of the five answers listed below are characteristics of water. When dissolved.
What is Dissolving? Answered Twinkl Teaching Wiki
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Four of the five answers listed below are characteristics of water. This process is called hydration,. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are..
How Does Salt Dissolve In Water Power Up Cook
Substances that are ____ give up hydrogen ions when they dissolve in water. Water is a polar molecule, which means it has a slightly. This process is called hydration,. In chemistry, it results in a solution, as the ionic bond of nacl. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that.
Dissolving science experiment with sugar dissolve in water 3333021
This process is called hydration,. When dissolved in water, a. Substances that are ____ give up hydrogen ions when they dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. A salt will dissolve in water to form.
Why does salt dissolve in water? AQuriousMind
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. When dissolved in water, a. This process is called hydration,. Water is a polar molecule, which.
Why does salt dissolve in water? AQuriousMind
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Substances that are ____ give up hydrogen ions when they dissolve in water. When dissolved in water, a. In chemistry, it results in a solution, as the ionic bond of nacl. Four of the five answers listed below are characteristics.
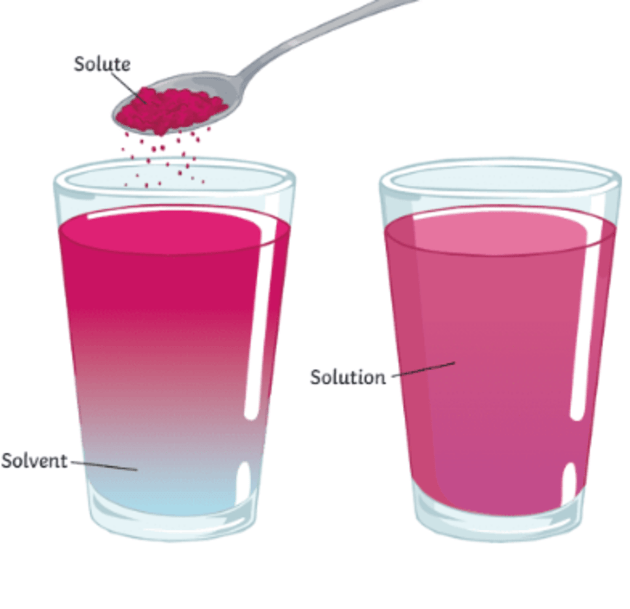
Solute Energy Education
At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Substances that are ____ give up hydrogen ions when they dissolve in water. Water is a polar molecule, which means it has a slightly. At the molecular level, salt dissolves in water due to electrical charges.
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik
When dissolved in water, a. This process is called hydration,. Salt dissolved in water is a rough description of earth's oceans. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. A salt will dissolve in water to form.
Is Dissolving Salt in Water a Chemical Change or a Physical Change
Substances that are ____ give up hydrogen ions when they dissolve in water. Water is a polar molecule, which means it has a slightly. Four of the five answers listed below are characteristics of water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are. Salt.
Four Of The Five Answers Listed Below Are Characteristics Of Water.
A salt will dissolve in water to form. When dissolved in water, a. Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl.
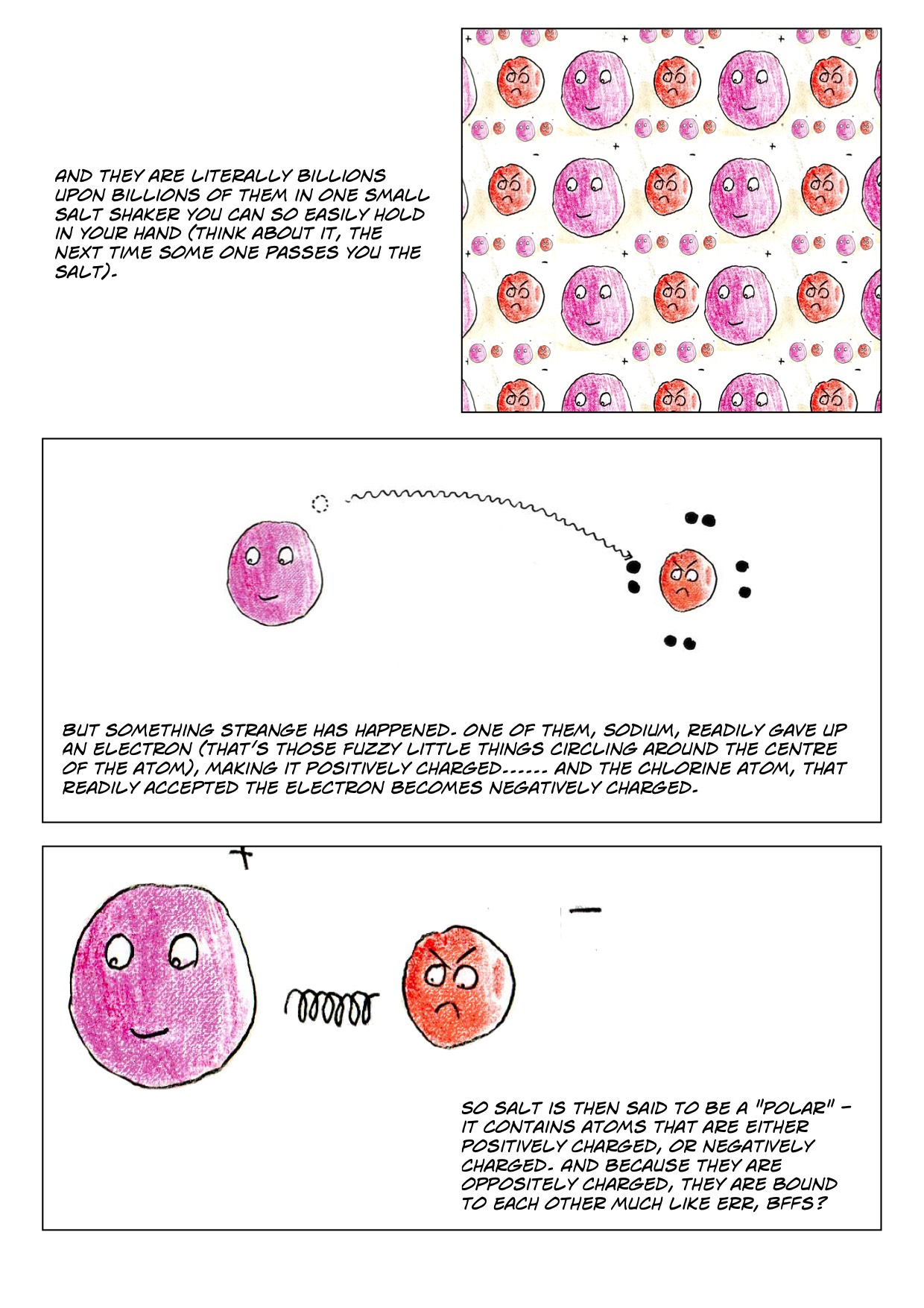
At The Molecular Level, Salt Dissolves In Water Due To Electrical Charges And Due To The Fact That Both Water And Salt Compounds Are.
Salt dissolves in water due to the polar nature of both substances. Water is a polar molecule, which means it has a slightly. This process is called hydration,. Substances that are ____ give up hydrogen ions when they dissolve in water.
At The Molecular Level, Salt Dissolves In Water Due To Electrical Charges And Due To The Fact That Both Water And Salt Compounds Are.
When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them.